CHEM 121
... Like the de Broglie relation, the Heisenberg Uncertainty Principle is important only for very small masses such as e-s, n0s, etc. §8-6 Quantum Mechanics & Schrödinger equation: Bohr model only applies to atoms with one eMotivated by classical wave equation for violin string ...
... Like the de Broglie relation, the Heisenberg Uncertainty Principle is important only for very small masses such as e-s, n0s, etc. §8-6 Quantum Mechanics & Schrödinger equation: Bohr model only applies to atoms with one eMotivated by classical wave equation for violin string ...
Quantum Numbers Handout File
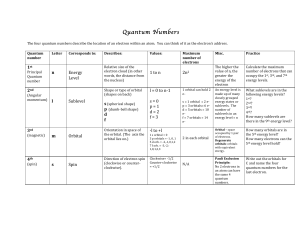
... What!sublevels!are!in!the! following!energy!levels?! 1st?! 2nd?! 3rd?! 4th?! How!many!sublevels!are! there!in!the!9th!energy!level?! ...
... What!sublevels!are!in!the! following!energy!levels?! 1st?! 2nd?! 3rd?! 4th?! How!many!sublevels!are! there!in!the!9th!energy!level?! ...
APCh7MB
... Electron affinity – the opposite of IE – change in energy when adding an electron 1. Across a period EA increases 2. Going down a group EA decreases Atomic radius – distance from nucleus to outermost electron 1. Across a period AR decreases Zeff 2. Going down a group AR increases A metal ion is + an ...
... Electron affinity – the opposite of IE – change in energy when adding an electron 1. Across a period EA increases 2. Going down a group EA decreases Atomic radius – distance from nucleus to outermost electron 1. Across a period AR decreases Zeff 2. Going down a group AR increases A metal ion is + an ...
107 chem Assement Q
... a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 state to the n = 2 state. 6. “It is impossible to know both the position and the momentum of an electron simultaneously” is a statement of: a. Hund’s Rule. b. deBroglie’s ...
... a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 state to the n = 2 state. 6. “It is impossible to know both the position and the momentum of an electron simultaneously” is a statement of: a. Hund’s Rule. b. deBroglie’s ...
2. Many-electron systems
... The angular part of the wave functions will be the SAME, i.e. Y (ϑ, ϕ). Therefore we can again classify the orbitals as 1s, 2s, 2p0 , 2p1 , 2p−1 , etc. The radial part: R(r) will differ, since the potential is different here form that of the H atom: since it is not a simple Coulomb-potencial, the de ...
... The angular part of the wave functions will be the SAME, i.e. Y (ϑ, ϕ). Therefore we can again classify the orbitals as 1s, 2s, 2p0 , 2p1 , 2p−1 , etc. The radial part: R(r) will differ, since the potential is different here form that of the H atom: since it is not a simple Coulomb-potencial, the de ...
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... Azimuthal and magnetic quantum numbers As angular momentum is a vector, one quantum number is related to its length, the other to its direction, in bound states the angular momentum is quantized as well. Spin and associated magnetic momentum of an electron ‘The Stern-Gerlach Experiment’ atoms passi ...
... Azimuthal and magnetic quantum numbers As angular momentum is a vector, one quantum number is related to its length, the other to its direction, in bound states the angular momentum is quantized as well. Spin and associated magnetic momentum of an electron ‘The Stern-Gerlach Experiment’ atoms passi ...
Chapter 3 notes
... Today’s Model!-Electron Cloud Today's model says electrons are not confined to ...
... Today’s Model!-Electron Cloud Today's model says electrons are not confined to ...
Figure 7.18 The 3d orbitals
... Led to 4 quantum numbers that describe the e-'s position in a complex equation: 1. Only certain wave functions are allowed 2. Each Ψn corresponds to an allowed energy for e- in atom 3. Thus energy of e- is quantized 4. Ψ has no physical meaning, but Ψ2 give the probability density 5. Allowed energy ...
... Led to 4 quantum numbers that describe the e-'s position in a complex equation: 1. Only certain wave functions are allowed 2. Each Ψn corresponds to an allowed energy for e- in atom 3. Thus energy of e- is quantized 4. Ψ has no physical meaning, but Ψ2 give the probability density 5. Allowed energy ...
Chapter 7
... orbitals of same shape but different position (ml = integers from –l to +l) • Spin quantum number, ms: indicates which of 2 possible spin states an electron is in, equal to either ...
... orbitals of same shape but different position (ml = integers from –l to +l) • Spin quantum number, ms: indicates which of 2 possible spin states an electron is in, equal to either ...
Document
... •the actual position of electrons can't really be specified •best we can do is say where they PROBABLY are •they are likely to be in cloud-like zones (called orbitals, not orbits) of varied shape •electrons with more energy can assume orbitals of increasingly bizzarre shape. These shapes sort of "fa ...
... •the actual position of electrons can't really be specified •best we can do is say where they PROBABLY are •they are likely to be in cloud-like zones (called orbitals, not orbits) of varied shape •electrons with more energy can assume orbitals of increasingly bizzarre shape. These shapes sort of "fa ...
Quantum Mechanical Model of the Atom
... The Compton Effect: When a high energy xray photon collides with a “free electron”, it gives some of its energy to the electron and a lower energy photon scatters off the electron. ...
... The Compton Effect: When a high energy xray photon collides with a “free electron”, it gives some of its energy to the electron and a lower energy photon scatters off the electron. ...
No Slide Title
... Chemistry in Action: Element from the Sun In 1868, Pierre Janssen detected a new dark line in the solar emission spectrum that did not match known emission lines ...
... Chemistry in Action: Element from the Sun In 1868, Pierre Janssen detected a new dark line in the solar emission spectrum that did not match known emission lines ...
Electromagnetic Radiation
... http://chemconnections.org/organicchem227/227assign-06.html#vision ...
... http://chemconnections.org/organicchem227/227assign-06.html#vision ...
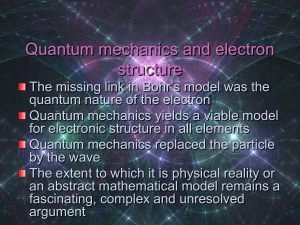
Quantum mechanics and electron structure
... Quantum mechanics and electron structure The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstr ...
... Quantum mechanics and electron structure The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstr ...