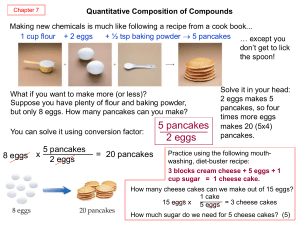
Chemical Reactions - 2012 Book Archive
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
indian association of chemistry teachers
... Website : www.careerpointgroup.com, Email: info@careerpointgroup.com ...
... Website : www.careerpointgroup.com, Email: info@careerpointgroup.com ...
Document
... – Atoms or molecules are passed into a beam of high‐speed electrons. – The high‐speed electrons knock electrons off the atoms or molecules being analyzed and change them to positive ions. – An applied electric field then accelerates these ions through a magnetic field, which deflects the paths o ...
... – Atoms or molecules are passed into a beam of high‐speed electrons. – The high‐speed electrons knock electrons off the atoms or molecules being analyzed and change them to positive ions. – An applied electric field then accelerates these ions through a magnetic field, which deflects the paths o ...
Alternative Coverage of moles, molarity, and Chemical Calculations
... Like atomic masses, molecular masses are relative masses. A molecule of oxygen, O2, has a mass of 32 u, twice that of a molecule of methane, 16 u. A molecule of ozone has a mass of 48 u, three times that of a molecule of methane. Using the same reasoning we used for atomic substances, we conclude th ...
... Like atomic masses, molecular masses are relative masses. A molecule of oxygen, O2, has a mass of 32 u, twice that of a molecule of methane, 16 u. A molecule of ozone has a mass of 48 u, three times that of a molecule of methane. Using the same reasoning we used for atomic substances, we conclude th ...
Document
... 1. Determine the mass in grams of each element present, if necessary. Remember, % means “out of 100”. 2. Convert grams of CO2 and H2O (or C and H) into moles of C and H atoms. 3. Convert moles of C into grams of C. Do the same for H. 4. Add masses for C and H and subtract the sum from the mass of t ...
... 1. Determine the mass in grams of each element present, if necessary. Remember, % means “out of 100”. 2. Convert grams of CO2 and H2O (or C and H) into moles of C and H atoms. 3. Convert moles of C into grams of C. Do the same for H. 4. Add masses for C and H and subtract the sum from the mass of t ...
Name:
... changes only slightly and therefore does not offset the increase in size due to the increase in energy levels. Atomic radius decreases as you go left to right across a period in the periodic table. The valence electrons are found in orbitals of the same energy level. At the same time, the effective ...
... changes only slightly and therefore does not offset the increase in size due to the increase in energy levels. Atomic radius decreases as you go left to right across a period in the periodic table. The valence electrons are found in orbitals of the same energy level. At the same time, the effective ...
Improving the Patch Transfer Function Approach for Fluid
... approaches is that they can be applied to any geometry and a wide range of mechanical properties. However, they are generally limited to the low frequency range, although the inexorable increase in computing capacities tends to extend their applicability to higher frequencies. This limitation of fre ...
... approaches is that they can be applied to any geometry and a wide range of mechanical properties. However, they are generally limited to the low frequency range, although the inexorable increase in computing capacities tends to extend their applicability to higher frequencies. This limitation of fre ...
Chapter 10
... Given two of the following, or information from which two of the following may be determined, calculate the third: theoretical yield, actual yield, percent yield. Theoretical Yield = Maximum amount of product possible based on amount of reactant(s). Actual Yield = How much product was actually ...
... Given two of the following, or information from which two of the following may be determined, calculate the third: theoretical yield, actual yield, percent yield. Theoretical Yield = Maximum amount of product possible based on amount of reactant(s). Actual Yield = How much product was actually ...
Analysis and Numerics of the Chemical Master Equation
... Each realisation is also independent of the other, allowing realisations to be run in parallel over as many cores as possible. Furthermore, the independence between realisations guarantees a linear speed up in computation time when using multi-core architectures. While the stochastic simulation meth ...
... Each realisation is also independent of the other, allowing realisations to be run in parallel over as many cores as possible. Furthermore, the independence between realisations guarantees a linear speed up in computation time when using multi-core architectures. While the stochastic simulation meth ...
Objectives - hartman
... Conversions of Quantities in Moles, continued Sample Problem A In a spacecraft, the carbon dioxide exhaled by astronauts can be removed by its reaction with lithium hydroxide, LiOH, according to the following chemical equation. CO2(g) + 2LiOH(s) → Li2CO3(s) + H2O(l) How many moles of lithium hydroxi ...
... Conversions of Quantities in Moles, continued Sample Problem A In a spacecraft, the carbon dioxide exhaled by astronauts can be removed by its reaction with lithium hydroxide, LiOH, according to the following chemical equation. CO2(g) + 2LiOH(s) → Li2CO3(s) + H2O(l) How many moles of lithium hydroxi ...
Chemistry - College of LAS
... students who require additional background before enrolling in CHEM 102. This course has been approved for graduation credit for all students in the College of LAS. Students in other colleges should check with their college office. Prerequisite: 2.5 years of high school mathematics, or credit or con ...
... students who require additional background before enrolling in CHEM 102. This course has been approved for graduation credit for all students in the College of LAS. Students in other colleges should check with their college office. Prerequisite: 2.5 years of high school mathematics, or credit or con ...
Chapter 3
... tion. The chemical equation must be made quantitatively correct without changing its qualitative chemical statement. Balancing a chemical equation requires something of a trial-and-error approach. You may find that you change the coefficient for a particular reactant or product, only to have to chan ...
... tion. The chemical equation must be made quantitatively correct without changing its qualitative chemical statement. Balancing a chemical equation requires something of a trial-and-error approach. You may find that you change the coefficient for a particular reactant or product, only to have to chan ...
Seven common errors in finding exact solutions of
... of nonlinear differential equations describing different processes in many scientific areas. The start of this science area was given in the famous work by Zabusky and Kruskal [1]. These authors showed that there are solitory waves with the property of the elastic particles in simple mathematical m ...
... of nonlinear differential equations describing different processes in many scientific areas. The start of this science area was given in the famous work by Zabusky and Kruskal [1]. These authors showed that there are solitory waves with the property of the elastic particles in simple mathematical m ...
1. Blood cholesterol levels are generally expressed as milligrams of
... increases which causes the increase in the volume of the helium balloon therefore it can blow up. therefore the material used to make the Helium balloon is foil type material which used to prevent the ...
... increases which causes the increase in the volume of the helium balloon therefore it can blow up. therefore the material used to make the Helium balloon is foil type material which used to prevent the ...
AS Chemistry - Edexcel
... References to third party material made in these sample assessment materials are made in good faith. Pearson does not endorse, approve or accept responsibility for the content of materials, which may be subject to change, or any opinions expressed therein. (Material may include textbooks, journals, ...
... References to third party material made in these sample assessment materials are made in good faith. Pearson does not endorse, approve or accept responsibility for the content of materials, which may be subject to change, or any opinions expressed therein. (Material may include textbooks, journals, ...
Mixed-space approach for calculation of vibration
... The phonon approach1,2 is currently the most efficient method for predicting thermodynamic properties of a solid at finite temperatures. It has been implemented under the framework of first-principles theories: the linear-response3,4 and supercell5,6 methods. In the literature, the supercell method is ...
... The phonon approach1,2 is currently the most efficient method for predicting thermodynamic properties of a solid at finite temperatures. It has been implemented under the framework of first-principles theories: the linear-response3,4 and supercell5,6 methods. In the literature, the supercell method is ...
Chapter 4 Classifying Reactions: Chemicals in Balance
... In each reaction below, a solid reacts with a gas to form a solid. Write a skeleton equation for each reaction. (a) carbon dioxide + calcium oxide → calcium carbonate (b) aluminum + oxygen → aluminum oxide (c) magnesium + oxygen → magnesium oxide What Is Required? You must write the skeleton equatio ...
... In each reaction below, a solid reacts with a gas to form a solid. Write a skeleton equation for each reaction. (a) carbon dioxide + calcium oxide → calcium carbonate (b) aluminum + oxygen → aluminum oxide (c) magnesium + oxygen → magnesium oxide What Is Required? You must write the skeleton equatio ...
Exam Review
... hotter parts of the tower, whereas those with lower boiling points condense near the cooler top of the tower. At various levels in the tower, trays collect mixtures of substances as they condense, each mixture containing compounds with similar boiling points. These mixtures are called petroleum fra ...
... hotter parts of the tower, whereas those with lower boiling points condense near the cooler top of the tower. At various levels in the tower, trays collect mixtures of substances as they condense, each mixture containing compounds with similar boiling points. These mixtures are called petroleum fra ...
Support Material
... Doping is the process of increasing the conductivity of intrinsic semiconductors by adding an appropriate amount of suitable impurity in Si or Ge. * n-type semiconductors : Silicon or Germinium (group-14) doped with electron rich impurity (group-15 element like P or As), Here conductivity is due to ...
... Doping is the process of increasing the conductivity of intrinsic semiconductors by adding an appropriate amount of suitable impurity in Si or Ge. * n-type semiconductors : Silicon or Germinium (group-14) doped with electron rich impurity (group-15 element like P or As), Here conductivity is due to ...
- Catalyst
... Plan: We need to determine the formula and the molecular mass from the atomic masses of each element multiplied by the subscripts. Solution: The formula is Na3PO4. The molar mass is 163.94 g/mol. Calculating the moles in a 38.6 g sample: moles of Na3PO4 = 38.6 g sample 1 mol 163.94 g sample = 0.235 ...
... Plan: We need to determine the formula and the molecular mass from the atomic masses of each element multiplied by the subscripts. Solution: The formula is Na3PO4. The molar mass is 163.94 g/mol. Calculating the moles in a 38.6 g sample: moles of Na3PO4 = 38.6 g sample 1 mol 163.94 g sample = 0.235 ...
Document
... weighted average of isotopes by their relative abundances. • lithium-6 (6.015 amu), which has a relative abundance of 7.50%, and • lithium-7 (7.016 amu), which has a relative abundance of 92.5%. ...
... weighted average of isotopes by their relative abundances. • lithium-6 (6.015 amu), which has a relative abundance of 7.50%, and • lithium-7 (7.016 amu), which has a relative abundance of 92.5%. ...