AP Chemistry Notes and Worksheets 2014
... Look at the image created for a sample of neon to the right. You can see three “peaks” or bumps on the graph. Each bump represents the amount of particles of that particular mass that were detected, telling us that there are three isotopes of neon in the sample. Example. Use the mass spectrum sh ...
... Look at the image created for a sample of neon to the right. You can see three “peaks” or bumps on the graph. Each bump represents the amount of particles of that particular mass that were detected, telling us that there are three isotopes of neon in the sample. Example. Use the mass spectrum sh ...
PDF of this page - Miami bulletin
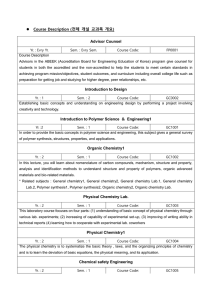
... Covers content similar to CHM 145. The focus of this laboratory course is for students with an interest in chemistry or biochemistry as a major. CAS-D/LAB. Prerequisite: CHM 144M. Co-requisite: CHM 142M. CHM 147. Introductory Seminar-Chemistry/Biochemistry. (1) An introduction to the various Chemist ...
... Covers content similar to CHM 145. The focus of this laboratory course is for students with an interest in chemistry or biochemistry as a major. CAS-D/LAB. Prerequisite: CHM 144M. Co-requisite: CHM 142M. CHM 147. Introductory Seminar-Chemistry/Biochemistry. (1) An introduction to the various Chemist ...
Changing Matter
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
Energy Foundations for High School Chemistry
... Preparing to Investigate What is energy? Most of us have a feeling that we understand energy and recognize it when we see it, but coming up with a formal definition might be harder for us to do. Here are some of the basic concepts associated with a definition of energy: • Energy is required to mak ...
... Preparing to Investigate What is energy? Most of us have a feeling that we understand energy and recognize it when we see it, but coming up with a formal definition might be harder for us to do. Here are some of the basic concepts associated with a definition of energy: • Energy is required to mak ...
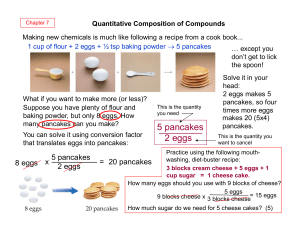
5 pancakes 2 eggs
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
guess paper class xii
... 15 How many ml of a 0.1M HCl are required to react completely with1 gm mixture of Na 2CO3 and NaHCO3 containing equimolar amounts of two? ...
... 15 How many ml of a 0.1M HCl are required to react completely with1 gm mixture of Na 2CO3 and NaHCO3 containing equimolar amounts of two? ...
CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the 'kinetic model' of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the 'kinetic model' of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
Computational Redox Potential Predictions Applications to Inorganic
... of these actinyl ions, which in turn alters the redox behavior of these actinyl ions in solution [15,16]. After the reduction process, either by the redox-active mineral surface, or by any organic reductants, for example quinones, or radicals, for example OH radicals, the actinyl(V) reduced species ...
... of these actinyl ions, which in turn alters the redox behavior of these actinyl ions in solution [15,16]. After the reduction process, either by the redox-active mineral surface, or by any organic reductants, for example quinones, or radicals, for example OH radicals, the actinyl(V) reduced species ...
Chapter 3 2014
... Al2(SO4)3 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 ...
... Al2(SO4)3 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... 3.6 Quantitative Information from Balanced Equations 1. A balanced reaction equation often provides more stoichiometric factors (or molar ratios) than needed to solve any particular stoichiometric problem. Often only one or two of them are relevant in a given problem. 2. The number of grams of reac ...
... 3.6 Quantitative Information from Balanced Equations 1. A balanced reaction equation often provides more stoichiometric factors (or molar ratios) than needed to solve any particular stoichiometric problem. Often only one or two of them are relevant in a given problem. 2. The number of grams of reac ...
Unit 3 Answer Key
... 3. You would have two times the Avogadro constant of hydrogen atoms. Rounded off, the number would be 2(6.02 × 1023) = 1.20 × 1024 hydrogen atoms. 4. You would not be able to see one person, but a mole of people is so many that they would be visible, as a group, from space. In fact, a mole of pe ...
... 3. You would have two times the Avogadro constant of hydrogen atoms. Rounded off, the number would be 2(6.02 × 1023) = 1.20 × 1024 hydrogen atoms. 4. You would not be able to see one person, but a mole of people is so many that they would be visible, as a group, from space. In fact, a mole of pe ...
High order schemes based on operator splitting and - HAL
... In the past decades high order and stable implicit Runge–Kutta schemes have been developed and widely investigated to solve stiff problems modeled by ODEs (see [32] Sect. IV and references therein). These advances can be naturally exploited to solve stiff semi–discrete problems originating from PDEs ...
... In the past decades high order and stable implicit Runge–Kutta schemes have been developed and widely investigated to solve stiff problems modeled by ODEs (see [32] Sect. IV and references therein). These advances can be naturally exploited to solve stiff semi–discrete problems originating from PDEs ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... – The reactant that is used up first in a reaction. – It controls the amounts of the other reactants that are used. – It controls the amount of product produced (maximum amount of product). – It produces less product than the other reactants possibly could produce. (***Use this for problem solving). ...
... – The reactant that is used up first in a reaction. – It controls the amounts of the other reactants that are used. – It controls the amount of product produced (maximum amount of product). – It produces less product than the other reactants possibly could produce. (***Use this for problem solving). ...
O - Montville.net
... 1 mole of O2 is need to react… 1. What relationships can be found in this equation? ...
... 1 mole of O2 is need to react… 1. What relationships can be found in this equation? ...
Stoichiometric Calculations
... Stoichiometric Calculations with Mass So far, we have seen stoichiometry problems that can be solved in one step, using only coefficients. ...
... Stoichiometric Calculations with Mass So far, we have seen stoichiometry problems that can be solved in one step, using only coefficients. ...
Chapter 3 Sem 2 2013-14
... Na2B4O7. You are given 20.0 g of borax...... (a) what is the formula mass of Na2B4O7 (b) how many moles of borax is 20.0 g? (c) how many moles of boron are present in 20.0 g Na2B4O7? (d) how many grams of boron are present in 20.0 g Na2B4O7? (e) how many atoms of B are present in 20.0g? (f) how many ...
... Na2B4O7. You are given 20.0 g of borax...... (a) what is the formula mass of Na2B4O7 (b) how many moles of borax is 20.0 g? (c) how many moles of boron are present in 20.0 g Na2B4O7? (d) how many grams of boron are present in 20.0 g Na2B4O7? (e) how many atoms of B are present in 20.0g? (f) how many ...
Answers
... the empirical formula, which shows only the simplest whole number ratio of one atom to another. It conveys the least information about a molecule. ...
... the empirical formula, which shows only the simplest whole number ratio of one atom to another. It conveys the least information about a molecule. ...
CHEM 121 Chp 5 Spaulding
... If 3.00 mol H2 reacts with 2.00 mol O2 how many moles of H2O will form? We just saw that H2 is the limiting reactant so 3.00 mol H2O will theoretically form If we run this in the lab and end up producing 1.75 mol H2O what is the percent yield? 1.75 mol H2O * 100% = 58.3 % 3.00 mol H2O ...
... If 3.00 mol H2 reacts with 2.00 mol O2 how many moles of H2O will form? We just saw that H2 is the limiting reactant so 3.00 mol H2O will theoretically form If we run this in the lab and end up producing 1.75 mol H2O what is the percent yield? 1.75 mol H2O * 100% = 58.3 % 3.00 mol H2O ...
Numerical solution of saddle point problems
... In the vast majority of cases, linear systems of saddle point type have real coefficients, and in this paper we restrict ourselves to the real case. Complex coefficient matrices, however, do arise in some cases; see, e.g., Bobrovnikova and Vavasis (2000), Mahawar and Sarin (2003) and Strang (1986, page ...
... In the vast majority of cases, linear systems of saddle point type have real coefficients, and in this paper we restrict ourselves to the real case. Complex coefficient matrices, however, do arise in some cases; see, e.g., Bobrovnikova and Vavasis (2000), Mahawar and Sarin (2003) and Strang (1986, page ...
Questa è la versione dell`autore dell`opera: [Chemical Reviews
... and adsorbate-surface interaction. The surface can be that of small crystallites or the internal surface of a porous system accessible to molecules. Radical formation is not uncommon during chemical processes taking place at surfaces. Radicals are usually intermediates of complex processes like, for ...
... and adsorbate-surface interaction. The surface can be that of small crystallites or the internal surface of a porous system accessible to molecules. Radical formation is not uncommon during chemical processes taking place at surfaces. Radicals are usually intermediates of complex processes like, for ...