Lab Manual (Eng. Medium)
... top (zero) calibration mark and then drained into a separate container until the calibration mark for the desired volume is reached. The remaining liquid is either discarded or returned to its original container. The maximum indicated capacity of some graduated pipettes is delivered by draining to a ...
... top (zero) calibration mark and then drained into a separate container until the calibration mark for the desired volume is reached. The remaining liquid is either discarded or returned to its original container. The maximum indicated capacity of some graduated pipettes is delivered by draining to a ...
Chapter 1 - Solutions
... reactant in predicting the amount of product obtained in a reaction? Can there be a limiting reactant if only one reactant is present? The limiting reactant is the reactant that first runs out in a chemical reaction, therefore limiting the amount of products that can be formed. Excess reactants refe ...
... reactant in predicting the amount of product obtained in a reaction? Can there be a limiting reactant if only one reactant is present? The limiting reactant is the reactant that first runs out in a chemical reaction, therefore limiting the amount of products that can be formed. Excess reactants refe ...
1.09 MB / 64 pages
... (a) Vitamins that are soluble in the fatty tissues in our bodies will tend to stay in the body for a substantial period of time. Vitamins that are soluble in water will dissolve in fluids like our blood plasma and can be transported to the kidneys, removed from the blood stream, and excreted. The fa ...
... (a) Vitamins that are soluble in the fatty tissues in our bodies will tend to stay in the body for a substantial period of time. Vitamins that are soluble in water will dissolve in fluids like our blood plasma and can be transported to the kidneys, removed from the blood stream, and excreted. The fa ...
Stoichiometry - Social Circle City Schools
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
physical setting chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Chapter 3 Stoichiometry
... that has been measured at the macroscopic level. This unit is called the mole, and it is based on quantities that we work with easily in the laboratory. For example, the eraser‐sized sample of aluminum that contains about 2 × 1022 Al atoms can also be described as containing 0.03 moles of Al atom ...
... that has been measured at the macroscopic level. This unit is called the mole, and it is based on quantities that we work with easily in the laboratory. For example, the eraser‐sized sample of aluminum that contains about 2 × 1022 Al atoms can also be described as containing 0.03 moles of Al atom ...
BRIEF ANSWERS TO SELECTED PROBLEMS APPENDIX G
... His measurements showed that a gas was involved in the reaction. He called this gas oxygen (one of his key discoveries). 1.16 A well-designed experiment must have the following essential features: (1) There must be at least two variables that are expected to be related; (2) there must be a way to co ...
... His measurements showed that a gas was involved in the reaction. He called this gas oxygen (one of his key discoveries). 1.16 A well-designed experiment must have the following essential features: (1) There must be at least two variables that are expected to be related; (2) there must be a way to co ...
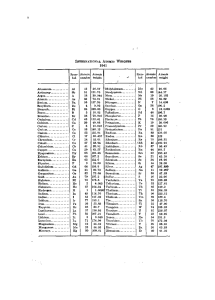
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
33 POLYMERS I OPTIONAL MODULE - 2
... like fibres and therefore can be woven into fabrics. The common examples are nylon66, dacron, silk, etc. 3. Thermoplastics : These are linear polymers with very few cross linkages or no cross linkages at all. The polymeric chains are held by weak VANDER WAAL forces and slide over one another. Due to ...
... like fibres and therefore can be woven into fabrics. The common examples are nylon66, dacron, silk, etc. 3. Thermoplastics : These are linear polymers with very few cross linkages or no cross linkages at all. The polymeric chains are held by weak VANDER WAAL forces and slide over one another. Due to ...
Chapter 8 PowerPoint - Southeast Online
... Making Molecules Mole-to-Mole Conversions • The balanced equation is the “recipe” for a chemical reaction. • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecul ...
... Making Molecules Mole-to-Mole Conversions • The balanced equation is the “recipe” for a chemical reaction. • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecul ...
Chemistry – A Molecular Sciences Appendices
... The molecular formula of a compound tells us how many atoms of each element are in one molecule. A carbon dioxide molecule, which has the formula CO2, contains one carbon atom and two oxygen atoms. This information is contained in the subscripts after each element. A molecule of sucrose (C12H22O11) ...
... The molecular formula of a compound tells us how many atoms of each element are in one molecule. A carbon dioxide molecule, which has the formula CO2, contains one carbon atom and two oxygen atoms. This information is contained in the subscripts after each element. A molecule of sucrose (C12H22O11) ...
Regularization Tools
... Singular values are discussed in detail in Section 2.3. Criterion 2 implies that the matrix A is ill-conditioned, i.e., that the solution is potentially very sensitive to perturbations; criterion 1 implies that there is no “nearby” problem with a well-conditioned coefficient matrix and with well-det ...
... Singular values are discussed in detail in Section 2.3. Criterion 2 implies that the matrix A is ill-conditioned, i.e., that the solution is potentially very sensitive to perturbations; criterion 1 implies that there is no “nearby” problem with a well-conditioned coefficient matrix and with well-det ...
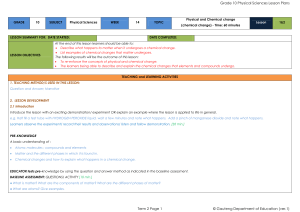
Physical Sciences Grade 10 Term 2
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
... tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To ...
Stoichiometry
... 2. Balance one element at a time using coefficients – Start with the elements in the most complex substance and finish with those in the least complex one – Alternatively, start with the element present in the fewest number of formulas and finish with the element present in the greatest number of fo ...
... 2. Balance one element at a time using coefficients – Start with the elements in the most complex substance and finish with those in the least complex one – Alternatively, start with the element present in the fewest number of formulas and finish with the element present in the greatest number of fo ...
Multiple Choice
... Acids contain the COOH functional group (B) (a is a ketone, c is an alcohol, and d is an ether) 4-methylpentane is the same as 2-methylpentane because # 4 C = # 2 (left to right vs. right to left). C1H3–C2C3H: C1 is sp3, C2 is sp, C3 is sp Both are non-polar, but CCl4 has more electrons ( more po ...
... Acids contain the COOH functional group (B) (a is a ketone, c is an alcohol, and d is an ether) 4-methylpentane is the same as 2-methylpentane because # 4 C = # 2 (left to right vs. right to left). C1H3–C2C3H: C1 is sp3, C2 is sp, C3 is sp Both are non-polar, but CCl4 has more electrons ( more po ...
2 - cloudfront.net
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
9278654 PS/Chemistry Ja03 - Dolgeville Central School
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
document
... Calculate the number of moles of oxygen required to react exactly with 4.3 moles of propane, C3H8, in the above reaction 4.3 moles of C3H8 requires how many moles of O2 There is a 1:5 ratio So 4.3(1) : 4.3(5) ...
... Calculate the number of moles of oxygen required to react exactly with 4.3 moles of propane, C3H8, in the above reaction 4.3 moles of C3H8 requires how many moles of O2 There is a 1:5 ratio So 4.3(1) : 4.3(5) ...
AP Chemistry - Siva Kodali
... at Fusion Learning Center and Fusion Academy. There, he enjoys convincing students that biology and chemistry are, in fact, fascinating journeys, not entirely designed to inflict pain on hapless teenagers. His military training occasionally aids him in this effort. He is the author of AP Biology For ...
... at Fusion Learning Center and Fusion Academy. There, he enjoys convincing students that biology and chemistry are, in fact, fascinating journeys, not entirely designed to inflict pain on hapless teenagers. His military training occasionally aids him in this effort. He is the author of AP Biology For ...