Midterm Review
... an enzyme, competing with the substrate – Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective ...
... an enzyme, competing with the substrate – Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective ...
BIOCHEMISTRY (CHEM 360)
... drive its transport. (4) The transport protein is needed to prevent the hydrolysis of the phospholipid chains as water crosses the membrane. ...
... drive its transport. (4) The transport protein is needed to prevent the hydrolysis of the phospholipid chains as water crosses the membrane. ...
File - Mrs Jones A
... Made of fatty acids and glycerol Glycerol can be converted to glucose, fatty acids can’t Contain many carbon atoms and hydrogen atoms SO SOURCE OF MANY PROTONS= Much ATP! What happens to the fatty acids?? ...
... Made of fatty acids and glycerol Glycerol can be converted to glucose, fatty acids can’t Contain many carbon atoms and hydrogen atoms SO SOURCE OF MANY PROTONS= Much ATP! What happens to the fatty acids?? ...
Document
... In animals and bacteria the extra step converts pyruvate to lactate (or lactic acid). This is a reduction, so NADH is used and NAD is regenerated, to be used in glycolysis. The reaction is reversible, so the energy remaining in the lactate molecule can be retrieved when oxygen becomes available and ...
... In animals and bacteria the extra step converts pyruvate to lactate (or lactic acid). This is a reduction, so NADH is used and NAD is regenerated, to be used in glycolysis. The reaction is reversible, so the energy remaining in the lactate molecule can be retrieved when oxygen becomes available and ...
Cellular Respiration
... carried out by plants, animals, and some bacteria – Anaerobic respiration: requires no oxygen and carried out by yeast, some bacteria, and sometimes animals ...
... carried out by plants, animals, and some bacteria – Anaerobic respiration: requires no oxygen and carried out by yeast, some bacteria, and sometimes animals ...
Cell Energy Part 3 – Respiration
... Low oxygen = body converts pyruvic acid into lactic acid = continued glycolysis After 1 to 3 minutes, lactic acid concentration in muscles is high High lactic acid = burning sensation in muscles & less glucose breakdown You stop = muscle damage is prevented Rest + oxygen = lactic acid pyruvic acid ...
... Low oxygen = body converts pyruvic acid into lactic acid = continued glycolysis After 1 to 3 minutes, lactic acid concentration in muscles is high High lactic acid = burning sensation in muscles & less glucose breakdown You stop = muscle damage is prevented Rest + oxygen = lactic acid pyruvic acid ...
Surface Infrared Spectroscopic Study of ATP Synthesis in Mitochondria
... When the same volume of the Pi-buffer was added to the mitochondrial suspension, no significant changes in IR spectra were observed around 1240 cm-1 [line (iii)]. These results indicate that the ADP→ATP conversion was successfully monitored with MIR-IRAS. Then we stopped oxygen supply and added an u ...
... When the same volume of the Pi-buffer was added to the mitochondrial suspension, no significant changes in IR spectra were observed around 1240 cm-1 [line (iii)]. These results indicate that the ADP→ATP conversion was successfully monitored with MIR-IRAS. Then we stopped oxygen supply and added an u ...
bioch8 - Otterville R
... Photosynthesis • Anabolic (small molecules combined) • Endergonic (stores energy) • Carbon dioxide (CO2) requiring process that uses light energy (photons) and water (H2O) to produce organic macromolecules (glucose). SUN photons ...
... Photosynthesis • Anabolic (small molecules combined) • Endergonic (stores energy) • Carbon dioxide (CO2) requiring process that uses light energy (photons) and water (H2O) to produce organic macromolecules (glucose). SUN photons ...
Chapter 6 ENZYME SUBSTRATE REACTANTS PRODUCTS
... These reactions occur in the cytoplasm.glycolysis and fermentation These are the three steps of cellular respiration. Acetyl coA formation, krebs, ETC This breaks glucose into two pyruvates.glycolysis This breaks acetyl CoA into carbon dioxide.krebs ATP is produced during these stages of glucose met ...
... These reactions occur in the cytoplasm.glycolysis and fermentation These are the three steps of cellular respiration. Acetyl coA formation, krebs, ETC This breaks glucose into two pyruvates.glycolysis This breaks acetyl CoA into carbon dioxide.krebs ATP is produced during these stages of glucose met ...
ATP
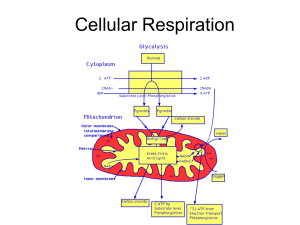
... The citric acid cycle For every turn of the cycle, one molecule of ATP and two molecules of carbon dioxide, 3 NADH, 1 FADH2 are produced. Makes a total of 2 ATP, 6 NADH, & 2 FADH2 Occurs in mitochondrial matrix Murdoch Online upload 2014 ...
... The citric acid cycle For every turn of the cycle, one molecule of ATP and two molecules of carbon dioxide, 3 NADH, 1 FADH2 are produced. Makes a total of 2 ATP, 6 NADH, & 2 FADH2 Occurs in mitochondrial matrix Murdoch Online upload 2014 ...
Cellular Respiration
... if we are deprived of oxygen for to long we will die because the cellular respiration reactions are dependent on the oxygen carrying molecule. Thus, without oxygen acceptors for carbon our bodies can’t get rid of the electrons that are bound to certain compounds. All compounds quickly use up the oxy ...
... if we are deprived of oxygen for to long we will die because the cellular respiration reactions are dependent on the oxygen carrying molecule. Thus, without oxygen acceptors for carbon our bodies can’t get rid of the electrons that are bound to certain compounds. All compounds quickly use up the oxy ...
PP Cellular Energy
... chemical bonds of the organic molecule that is broken down. • Cellular respiration involves many different reactions, each controlled by its own enzyme. • Cellular respiration usually uses glucose however fats (fatty acids and glycerol) and amino acids can also be used. ...
... chemical bonds of the organic molecule that is broken down. • Cellular respiration involves many different reactions, each controlled by its own enzyme. • Cellular respiration usually uses glucose however fats (fatty acids and glycerol) and amino acids can also be used. ...
Name: Per
... 4. What gas builds up inside the plant during these conditions? 5. When oxygen is added to RuBP, instead of carbon (from carbon dioxide), can sugar be made? 6. Enzyme that adds carbon or oxygen to RuBP (rubisco) binds with oxygen more favorably than CO2. Oxygen and carbon dioxide can both fit in rub ...
... 4. What gas builds up inside the plant during these conditions? 5. When oxygen is added to RuBP, instead of carbon (from carbon dioxide), can sugar be made? 6. Enzyme that adds carbon or oxygen to RuBP (rubisco) binds with oxygen more favorably than CO2. Oxygen and carbon dioxide can both fit in rub ...
Chapter 5 Active Lecture Questions
... Apoenzymes are inactive by themselves and must be activated by ...
... Apoenzymes are inactive by themselves and must be activated by ...
CH 2. CELLULAR RESPIRATION
... Since the activation energy needed for the combustion of glucose is quite high, each step in cellular respiration is catalyzed by specific enzymes that lower the activation energies and allow the reactions to occur at a pace fast enough to maintain cell needs. ...
... Since the activation energy needed for the combustion of glucose is quite high, each step in cellular respiration is catalyzed by specific enzymes that lower the activation energies and allow the reactions to occur at a pace fast enough to maintain cell needs. ...
Comprehenexam- - HCC Learning Web
... 30) Plants store carbohydrate as a polymer called _______________ while animals store carbohydrates as a polymer called _________________ 31) How will you distinguish between hydrolysis and dehydration reactions? Give an example each. _______________________________________________________________ ...
... 30) Plants store carbohydrate as a polymer called _______________ while animals store carbohydrates as a polymer called _________________ 31) How will you distinguish between hydrolysis and dehydration reactions? Give an example each. _______________________________________________________________ ...
Energy For Movement - Illinois Wesleyan University
... be converted to glucose-6phosphate before it can be used for energy. For glucose this process takes 1 ATP. Glycolysis ultimately produces pyruvic acid which is then converted to lactic acid in the absence of oxygen. Gycolysis requires 12 enzymatic reactions to form lactic acid which occur within ...
... be converted to glucose-6phosphate before it can be used for energy. For glucose this process takes 1 ATP. Glycolysis ultimately produces pyruvic acid which is then converted to lactic acid in the absence of oxygen. Gycolysis requires 12 enzymatic reactions to form lactic acid which occur within ...
103 topic summary
... hydrogen bonding and disulfide bonds Globular vs. fibrous proteins Protein hydrolysis (enzymes and resistance to acid hydrolysis) Denaturation of proteins: heat, acids and bases, organic compounds, heavy metal ions and agitation Chapter 21: Enzymes as catalysts (effects on activation energy and reac ...
... hydrogen bonding and disulfide bonds Globular vs. fibrous proteins Protein hydrolysis (enzymes and resistance to acid hydrolysis) Denaturation of proteins: heat, acids and bases, organic compounds, heavy metal ions and agitation Chapter 21: Enzymes as catalysts (effects on activation energy and reac ...
Fatty Acid Catabolism - Chemistry Courses: About
... • Opposite of beta oxidation in the sense that 2carbon acetate units are linked to form evenchain, saturated fatty acids • Differs from Fatty acid degradation – In cytoplasm, not matrix – Acyl carrier protein rather than CoA – Enzymes linked in a complex – Utilizes NADPH – Energetically linked to AT ...
... • Opposite of beta oxidation in the sense that 2carbon acetate units are linked to form evenchain, saturated fatty acids • Differs from Fatty acid degradation – In cytoplasm, not matrix – Acyl carrier protein rather than CoA – Enzymes linked in a complex – Utilizes NADPH – Energetically linked to AT ...
Title - Iowa State University
... 11. Which of the following best describes the process that occurs during the citric acid cycle? a. Pyruvate is processed to release one molecule of carbon dioxide, and the remaining carbons are used to form acetyl CoA. b. One molecule of glucose is broken into two molecules of pyruvate, ATP is produ ...
... 11. Which of the following best describes the process that occurs during the citric acid cycle? a. Pyruvate is processed to release one molecule of carbon dioxide, and the remaining carbons are used to form acetyl CoA. b. One molecule of glucose is broken into two molecules of pyruvate, ATP is produ ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.