Ch.7 Summary Notes
... Radiocarbon dating is the process of determining the age of an object by measuring the amount of carbon-14 remaining in that object. Carbon’s isotopes include carbon-12 and carbon14. When an organism is alive, the ratio of carbon- 14 atoms to carbon-12 atoms in the organism remains nearly constant. ...
... Radiocarbon dating is the process of determining the age of an object by measuring the amount of carbon-14 remaining in that object. Carbon’s isotopes include carbon-12 and carbon14. When an organism is alive, the ratio of carbon- 14 atoms to carbon-12 atoms in the organism remains nearly constant. ...
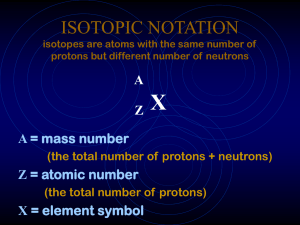
ISOTOPIC NOTATION isotopes are atoms with the same number
... An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
... An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
Distinguishing Among Atoms Worksheet
... relative abundance of its isotopes. a. In nature, most elements occur as a mixture of two or more isotopes. b. Isotopes of an element do not have a specific natural percent abundance. c. The average atomic mass of an element is usually closest to that of the isotope with the highest natural abundanc ...
... relative abundance of its isotopes. a. In nature, most elements occur as a mixture of two or more isotopes. b. Isotopes of an element do not have a specific natural percent abundance. c. The average atomic mass of an element is usually closest to that of the isotope with the highest natural abundanc ...
Course 2.2. Organic matter
... Theoretically: NH4+ + 2 O2 NO3- + 2H+ + H2O Nitrogen Oxygen Demand (nBOD) = 4.57 g O2 / g NH4-N ! When no measures are taken to prevent this, the analysis result is called TBOD (total BOD). When a so-called nitrification inhibitor is added (prevents conversion of NH4+), then it is called CBOD (car ...
... Theoretically: NH4+ + 2 O2 NO3- + 2H+ + H2O Nitrogen Oxygen Demand (nBOD) = 4.57 g O2 / g NH4-N ! When no measures are taken to prevent this, the analysis result is called TBOD (total BOD). When a so-called nitrification inhibitor is added (prevents conversion of NH4+), then it is called CBOD (car ...
Biogeochemical Cycles Note Slides File
... – If farmers plant legumes, they will also have bacteria in the soil that perform nitrogen fixation. – This helps reduce fertilizer use! ...
... – If farmers plant legumes, they will also have bacteria in the soil that perform nitrogen fixation. – This helps reduce fertilizer use! ...
Oxygen - Matheson
... Applications Oxygen is generally liquefied so that it can be more effectively transported and stored in large volumes. However, most applications use oxygen after it is vaporized to the gaseous form. The primary uses of oxygen relate to its strong oxidizing and lifesustaining properties. Oxygen is c ...
... Applications Oxygen is generally liquefied so that it can be more effectively transported and stored in large volumes. However, most applications use oxygen after it is vaporized to the gaseous form. The primary uses of oxygen relate to its strong oxidizing and lifesustaining properties. Oxygen is c ...
In-Situ Chlorine-36
... First and foremost, determine the rock/mineral type you want to sample, from what surface and how many samples you need to collect. Because chlorine-36 is produced from several target elements, virtually all rock types are suitable for sampling. The number of samples is related to geological charact ...
... First and foremost, determine the rock/mineral type you want to sample, from what surface and how many samples you need to collect. Because chlorine-36 is produced from several target elements, virtually all rock types are suitable for sampling. The number of samples is related to geological charact ...
Unit 3 Summary Booklet
... Radioisotopes have uses in the home, in health and in industry. Home: Smoke alarms contain the radioisotope Americium-241. Health: Cancer can be treated using the radioisotope Iodine-131 Industry: Radioisotopes can be used to gauge the thickness of materials. The fear of radioactivity damaging ...
... Radioisotopes have uses in the home, in health and in industry. Home: Smoke alarms contain the radioisotope Americium-241. Health: Cancer can be treated using the radioisotope Iodine-131 Industry: Radioisotopes can be used to gauge the thickness of materials. The fear of radioactivity damaging ...
worksheet #1 - chemistryrocks.net
... tables are “weighted averages” of the weights of the different naturally occurring isotopes of the element. Let’s look at an example. Approximately 75% of the chlorine atoms found in nature have a mass of 35. The other 25% have a mass of 37. What should we report as the atomic weight for chlorine? W ...
... tables are “weighted averages” of the weights of the different naturally occurring isotopes of the element. Let’s look at an example. Approximately 75% of the chlorine atoms found in nature have a mass of 35. The other 25% have a mass of 37. What should we report as the atomic weight for chlorine? W ...
Make a large atom with p:95, n:146, e:95 - TSDCurriculum
... 5. READ: This simulation only lets you to build atoms that exist in nature or have been made by scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. ...
... 5. READ: This simulation only lets you to build atoms that exist in nature or have been made by scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. ...
atom
... nature, most elements are found combined with other elements in compounds. A chemical compound is a substance formed by the chemical combination of two or more elements in definite proportions. The physical and chemical properties of a compound are different from the elements from which it is fo ...
... nature, most elements are found combined with other elements in compounds. A chemical compound is a substance formed by the chemical combination of two or more elements in definite proportions. The physical and chemical properties of a compound are different from the elements from which it is fo ...
Solutions 1a (suggested problems before Exam #1) Chem151 [Kua
... Molecular pictures must show that atoms of every element are conserved. The solid-gas transformation is correctly represented, but the number of molecules is not conserved. Here is one way to show a correct picture: ...
... Molecular pictures must show that atoms of every element are conserved. The solid-gas transformation is correctly represented, but the number of molecules is not conserved. Here is one way to show a correct picture: ...
Atoms - Chemistry Land
... Electrons are not the simple little particles that we often draw them. They demonstrate the dual nature of matter, which is both particle and wave. There shape or location is quite flexible. In this shape there are two lobes. The electron occupies both. Somehow is gets back and forth without passin ...
... Electrons are not the simple little particles that we often draw them. They demonstrate the dual nature of matter, which is both particle and wave. There shape or location is quite flexible. In this shape there are two lobes. The electron occupies both. Somehow is gets back and forth without passin ...
File
... 14. Atoms W, X, Y, and Z have the following nuclear compositions. Which two are isotopes? How do you know? ...
... 14. Atoms W, X, Y, and Z have the following nuclear compositions. Which two are isotopes? How do you know? ...
Chemistry 515 Name: L. S. Curtin Soc. Sec. #: February 8, 1999
... Elements in compounds combine in fixed percentages by mass not volume. 2) Which of the following statements is correct? a) The number of protons and neutrons in the nucleus of an atom are always equal. b) The mass of an atom is contained primarily in the nucleus and the volume of an atom is primaril ...
... Elements in compounds combine in fixed percentages by mass not volume. 2) Which of the following statements is correct? a) The number of protons and neutrons in the nucleus of an atom are always equal. b) The mass of an atom is contained primarily in the nucleus and the volume of an atom is primaril ...
Chapter 4 Section 4.3
... write the chemical name followed by the mass number (protons + neutrons) ...
... write the chemical name followed by the mass number (protons + neutrons) ...
b. Elements as Mixtures - Isotopes
... Elements are made from atoms having the same atomic number, protons Are all atoms of one particular atom the same or are they mixtures? 1) All atom nuclei for an element have the same number of protons. 2) Every atom in an element has the same number of protons & electrons 3) However, elements are ...
... Elements are made from atoms having the same atomic number, protons Are all atoms of one particular atom the same or are they mixtures? 1) All atom nuclei for an element have the same number of protons. 2) Every atom in an element has the same number of protons & electrons 3) However, elements are ...
Isotopes File - Northwest ISD Moodle
... When it comes to fighting cancer, doctors face two questions. Where has the cancer spread? And how can it be treated? In the case of one type of cancer, the answer to both questions may involve the isotope iodine-131. Iodine-131 is an artificial isotope of iodine. This isotope releases radiation tha ...
... When it comes to fighting cancer, doctors face two questions. Where has the cancer spread? And how can it be treated? In the case of one type of cancer, the answer to both questions may involve the isotope iodine-131. Iodine-131 is an artificial isotope of iodine. This isotope releases radiation tha ...
Review of Moles and Stoichiometry
... 13.) A sample of urea contains 1.121 g N, 0.161 g H, 0.480 g C, and 0.640 g O. a.) By mass, what percent of urea is C and what percent is O? ...
... 13.) A sample of urea contains 1.121 g N, 0.161 g H, 0.480 g C, and 0.640 g O. a.) By mass, what percent of urea is C and what percent is O? ...
Name Honors Chemistry ___/___/___ Subatomic Particles Atomic
... The atomic weight of an element is the weighted average of the masses of the isotopes of that element. The weighted average is determined using the abundance and mass of each isotope. Most elements have more than one naturally occurring isotope. For example, there are two naturally occurring isotope ...
... The atomic weight of an element is the weighted average of the masses of the isotopes of that element. The weighted average is determined using the abundance and mass of each isotope. Most elements have more than one naturally occurring isotope. For example, there are two naturally occurring isotope ...
Atoms
... •Isotopes are atoms of the same element that differ in the number of neutrons. Isotopes of the same element have the same chemical properties, because they have the same number of protons and electrons. Isotopes are identified by mass number. Neutrons affect mass, so, isotopes with more neutrons ar ...
... •Isotopes are atoms of the same element that differ in the number of neutrons. Isotopes of the same element have the same chemical properties, because they have the same number of protons and electrons. Isotopes are identified by mass number. Neutrons affect mass, so, isotopes with more neutrons ar ...
North Haven Public Schools Curriculum
... Thomson, Bohr, Rutherford, and Millikan. The position of an element in the periodic table is related to its atomic number. Conservation of Matter and Stoichiometry – The conservation of atoms in chemical reactions leads to the principle of conservations of matter and the ability to calculate the m ...
... Thomson, Bohr, Rutherford, and Millikan. The position of an element in the periodic table is related to its atomic number. Conservation of Matter and Stoichiometry – The conservation of atoms in chemical reactions leads to the principle of conservations of matter and the ability to calculate the m ...
X1-1 - murov.info
... Answer the following using only a periodic table as a source of information. Give as much information as possible using only the atomic number and atomic mass. 1. How many protons, neutrons and electrons are in a.* F b. Al c. Mn d. Au 2. How many protons, neutrons and electrons are in a.* Cl b. Cu 3 ...
... Answer the following using only a periodic table as a source of information. Give as much information as possible using only the atomic number and atomic mass. 1. How many protons, neutrons and electrons are in a.* F b. Al c. Mn d. Au 2. How many protons, neutrons and electrons are in a.* Cl b. Cu 3 ...
Isotope analysis

Isotope analysis is the identification of isotopic signature, the distribution of certain stable isotopes and chemical elements within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level, and subsistence. Variations in isotope ratios from isotopic fractionation are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.The ratios of isotopic oxygen are also differentially affected by global weather patterns and regional topography as moisture is transported. Areas of lower humidity cause the preferential loss of 18O water in the form of vapor and precipitation. Furthermore, evaporated 16O water returns preferentially to the atmospheric system as it evaporates and 18O remains in liquid form or is incorporated into the body water of plants and animals.