No Slide Title
... • Cu is called the reducing agent because it caused Ag+ to be reduced; and Ag+ is called the oxidizing agent because it caused Cu to be oxidized. ...
... • Cu is called the reducing agent because it caused Ag+ to be reduced; and Ag+ is called the oxidizing agent because it caused Cu to be oxidized. ...
Chapter 11 Chemical Reactions
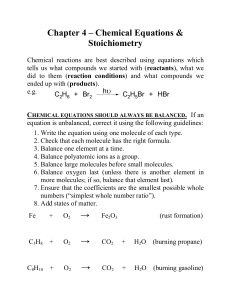
... 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
... 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
Chemical Reactions and Equations
... Reaction is a term used for depicting a change or transformation in which a substance decomposes, combines with other substances, or interchanges constituents with other substances. What is a ‘Chemical Reaction’? A chemical change is always accompanied by a chemical reaction. a chemical change or re ...
... Reaction is a term used for depicting a change or transformation in which a substance decomposes, combines with other substances, or interchanges constituents with other substances. What is a ‘Chemical Reaction’? A chemical change is always accompanied by a chemical reaction. a chemical change or re ...
Take notes on this document while you are watching the recorded
... A. Minerals: Are defined depending on the context. Any natural, inorganic molecule might be defined as a mineral (therefore, some include water and phosphate). However, most often, when we discuss minerals in nutrition, we are talking about those inorganic substances in pure, or elemental, form (Cal ...
... A. Minerals: Are defined depending on the context. Any natural, inorganic molecule might be defined as a mineral (therefore, some include water and phosphate). However, most often, when we discuss minerals in nutrition, we are talking about those inorganic substances in pure, or elemental, form (Cal ...
CHM1 Review for Exam 9 Topics 1. Reaction Types a. Combustion
... When the equation is correctly balanced using the smallest wholenumber coefficients, what is the coefficient of CO? ...
... When the equation is correctly balanced using the smallest wholenumber coefficients, what is the coefficient of CO? ...
File
... into reactants in order for their bonds to break, that minium enegy is called activation energy. The Relationship Between Kinetic (K.E.) and Potential Energy (P.E) Potential energy is defined as energy of position. Energy is conserved in chemical and physical changes. That means kinetic energy (ener ...
... into reactants in order for their bonds to break, that minium enegy is called activation energy. The Relationship Between Kinetic (K.E.) and Potential Energy (P.E) Potential energy is defined as energy of position. Energy is conserved in chemical and physical changes. That means kinetic energy (ener ...
PERIODIC TABLE
... 43- The percentage of carbon atom for a compound is (60.87%), which of the formula is the empirical formula a- (C7H6O4) b- (C7H6O3) c- (C7H6O2) d- (C7H6O) 44- When two s-atomic-orbitals form a linear combination, the molecular orbital obtained, is:: a- s b- p c- σ d- π 45- In 0.500 mol of Na2CO3 10 ...
... 43- The percentage of carbon atom for a compound is (60.87%), which of the formula is the empirical formula a- (C7H6O4) b- (C7H6O3) c- (C7H6O2) d- (C7H6O) 44- When two s-atomic-orbitals form a linear combination, the molecular orbital obtained, is:: a- s b- p c- σ d- π 45- In 0.500 mol of Na2CO3 10 ...
Chapter 5 - U of L Class Index
... number of collisions between molecules and also provide the collisions with the required energy of activation. Raising the temperature almost always increases the rate of reaction. Conversely, lowering the temperature will reduce the rate of reaction. Concentration. The rate of reaction increases wh ...
... number of collisions between molecules and also provide the collisions with the required energy of activation. Raising the temperature almost always increases the rate of reaction. Conversely, lowering the temperature will reduce the rate of reaction. Concentration. The rate of reaction increases wh ...
Supramolecular catalysis

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.A closely related field of study is asymmetric catalysis which requires molecular recognition to differentiate two chiral starting material or chiral transition states and thus it could be categorized as an area of supramolecular catalysis, but supramolecular catalysis however does not necessarily have to involve asymmetric reaction. As there is another Wikipedia article already written about small molecule asymmetric catalysts, this article focuses primarily on large catalytic host molecules. Non-discrete and structurally poorly defined system such as micelle and dendrimers are not included.