Catalyst Notes - University of Idaho
... increase reaction rates to the same extent in both the forward and reverse directions do not modify the Gibbs free energy change are not consumed in the reaction concentration cancels out in the calculation of the equilibrium constant a small amount of catalyst affects the rate of reaction for a lar ...
... increase reaction rates to the same extent in both the forward and reverse directions do not modify the Gibbs free energy change are not consumed in the reaction concentration cancels out in the calculation of the equilibrium constant a small amount of catalyst affects the rate of reaction for a lar ...
Name - rwebbchem
... with AgNO3, Pb(NO3)2, and BaCl2. Precipitates form in all three cases. Which of the following could be the anion of the unknown salt: Br-, CO32-, or NO3-? ...
... with AgNO3, Pb(NO3)2, and BaCl2. Precipitates form in all three cases. Which of the following could be the anion of the unknown salt: Br-, CO32-, or NO3-? ...
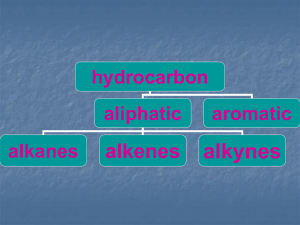
Methane - ARZELORIVAS IS
... This reaction has the following characteristic properties. It doesn't take place in the dark or at low temperatures. It occurs in the presence of ultraviolet light or at temperatures above 250oC. Once the reaction gets started, it continues after the light is turned off. The products of the ...
... This reaction has the following characteristic properties. It doesn't take place in the dark or at low temperatures. It occurs in the presence of ultraviolet light or at temperatures above 250oC. Once the reaction gets started, it continues after the light is turned off. The products of the ...
O 2
... Synthesis is also known as direct combination; it is the combining together of several elements and/or compounds into one new compound. Synthesis takes the general format: ...
... Synthesis is also known as direct combination; it is the combining together of several elements and/or compounds into one new compound. Synthesis takes the general format: ...
Fun With Predicting Reaction Products
... reaction to occur, both reactants and only one of the products must be soluble in water. If you look up the solubilities on a chart, you’ll find that Ag2SO3 is partly soluble in water, and all of the other compounds are totally soluble in water. This tells us that this reaction will not occur. ...
... reaction to occur, both reactants and only one of the products must be soluble in water. If you look up the solubilities on a chart, you’ll find that Ag2SO3 is partly soluble in water, and all of the other compounds are totally soluble in water. This tells us that this reaction will not occur. ...
Review of Thermodynamics
... This set of archetypal interactions can be likened to a set of ingredients that when combined create a menu that we observe as molecular recognition properties of molecules. Bringing two or more molecules together results in preferences for particular orientations that can lead to particular reactiv ...
... This set of archetypal interactions can be likened to a set of ingredients that when combined create a menu that we observe as molecular recognition properties of molecules. Bringing two or more molecules together results in preferences for particular orientations that can lead to particular reactiv ...
chemistry 102 fall 2001 part 1
... (3) Do NOT write on the envelope. (4) Bubble in OPTION A on the scanning sheet IF you want your grade posted. (5) When finished, put the free response answers in the envelope with the scanning sheet. You can keep the multiple choice part - the answers will be given to you as you leave. (6) There are ...
... (3) Do NOT write on the envelope. (4) Bubble in OPTION A on the scanning sheet IF you want your grade posted. (5) When finished, put the free response answers in the envelope with the scanning sheet. You can keep the multiple choice part - the answers will be given to you as you leave. (6) There are ...
2012 Chem 13 News Exam
... Starting from reactants only, and before equilibrium is established, the rate of the forward reaction continually increases. ...
... Starting from reactants only, and before equilibrium is established, the rate of the forward reaction continually increases. ...
Reactions Unit Plan
... How does matter chemically change? How can chemical changes be described and predicted? ...
... How does matter chemically change? How can chemical changes be described and predicted? ...
Supramolecular catalysis

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.A closely related field of study is asymmetric catalysis which requires molecular recognition to differentiate two chiral starting material or chiral transition states and thus it could be categorized as an area of supramolecular catalysis, but supramolecular catalysis however does not necessarily have to involve asymmetric reaction. As there is another Wikipedia article already written about small molecule asymmetric catalysts, this article focuses primarily on large catalytic host molecules. Non-discrete and structurally poorly defined system such as micelle and dendrimers are not included.