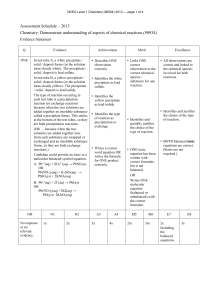
The effect of confinement on chemical reactions
... The results in Fig. 4 show that there is a significant enhancement of the equilibrium yield for the confined fluid, as the mole fraction of the ester is more than doubled in the pore phase. This difference was shown [5] to be due to the selective adsorption of the ester, which has a more favorable f ...
... The results in Fig. 4 show that there is a significant enhancement of the equilibrium yield for the confined fluid, as the mole fraction of the ester is more than doubled in the pore phase. This difference was shown [5] to be due to the selective adsorption of the ester, which has a more favorable f ...
Second Semester Review Part 1
... proceeds by bimolecular collisions between A2 and B2 molecules. If the concentrations of both A2 and B2 are doubled, the reaction rate will be changed by a factor of (A) ½ ...
... proceeds by bimolecular collisions between A2 and B2 molecules. If the concentrations of both A2 and B2 are doubled, the reaction rate will be changed by a factor of (A) ½ ...
2014 Academic Challenge Sectional Chemistry Exam Solution Set 1
... B. Only the second molecule is polar. In the third and fourth structures, the square planar shape causes any bond dipoles to cancel out. ...
... B. Only the second molecule is polar. In the third and fourth structures, the square planar shape causes any bond dipoles to cancel out. ...
Modeling CO Oxidation on Silica-Supported Iron Oxide under
... was shown that, after a period under Ar, the rate of oxidation of the catalyst by N2O is higher than the rate at the end of the previous period under N2O. This phenomena was shown to occur through diffusion of oxygen abstracted from N2O at the surface of the catalyst into subsurface oxygen vacancies ...
... was shown that, after a period under Ar, the rate of oxidation of the catalyst by N2O is higher than the rate at the end of the previous period under N2O. This phenomena was shown to occur through diffusion of oxygen abstracted from N2O at the surface of the catalyst into subsurface oxygen vacancies ...
F Practice Test #2 Solutions
... your records, you may want to mark your answers on this sheet. On the Scantron you need to fill in your perm number, test version, and name. Failure to do any of these things will result in the loss of 1 point. Your perm number is placed and bubbled in under the “ID number”. Do not skip boxes or put ...
... your records, you may want to mark your answers on this sheet. On the Scantron you need to fill in your perm number, test version, and name. Failure to do any of these things will result in the loss of 1 point. Your perm number is placed and bubbled in under the “ID number”. Do not skip boxes or put ...
AP CHEMISTRY PROBLEMS ENTHALPY, ENTROPY, AND FREE
... 4. For each of the following pairs of substances, which one has the greatest value of ΔS at 25 °C? a. C graphite or C diamond b. C2H5OH (l) or C2H5OH (g) c. CO2 (s) or CO2 (g) d. N2O at 0 K, or He at 10 K e. N2O(g) at 1 atm, 25 °C; or He(g) at 1 atm and 25 °C f. H2O (s) at 0 °C or H2O (l) at 0 °C 5. ...
... 4. For each of the following pairs of substances, which one has the greatest value of ΔS at 25 °C? a. C graphite or C diamond b. C2H5OH (l) or C2H5OH (g) c. CO2 (s) or CO2 (g) d. N2O at 0 K, or He at 10 K e. N2O(g) at 1 atm, 25 °C; or He(g) at 1 atm and 25 °C f. H2O (s) at 0 °C or H2O (l) at 0 °C 5. ...
A reaction - 固体表面物理化学国家重点实验室
... (a) Derive the integrated rate equation for a reaction of 3/2 order. Derive the expression for the half-life of such a reaction. Can you think of an example of such a reaction? (b) Derive the integrated rate equation for a reaction of order n. 2. The reaction NO3 + NO 2NO2 is known as an elementar ...
... (a) Derive the integrated rate equation for a reaction of 3/2 order. Derive the expression for the half-life of such a reaction. Can you think of an example of such a reaction? (b) Derive the integrated rate equation for a reaction of order n. 2. The reaction NO3 + NO 2NO2 is known as an elementar ...
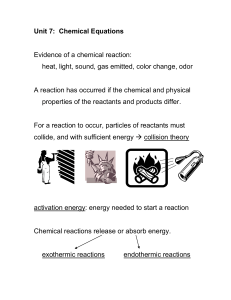
Using thermodynamics, we can predict whether or not a reaction will
... enter into the reaction--they only provide a surface for the reaction to take place on and serves to weaken the bonds of one of the reactants which adsorbs to it. This then makes it easier for the other reactant to react with the one with weak bonds and thus the reaction rate is faster. The catalyti ...
... enter into the reaction--they only provide a surface for the reaction to take place on and serves to weaken the bonds of one of the reactants which adsorbs to it. This then makes it easier for the other reactant to react with the one with weak bonds and thus the reaction rate is faster. The catalyti ...
Equilibrium
... When the rates of the forward and reverse reactions are equal, the reaction has reached a state of balance called chemical equilibrium. At chemical equilibrium, no net change occurs in the actual amounts of the components of the system. The amount of SO3 in the equilibrium mixture is the maximum amo ...
... When the rates of the forward and reverse reactions are equal, the reaction has reached a state of balance called chemical equilibrium. At chemical equilibrium, no net change occurs in the actual amounts of the components of the system. The amount of SO3 in the equilibrium mixture is the maximum amo ...
Supramolecular catalysis

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.A closely related field of study is asymmetric catalysis which requires molecular recognition to differentiate two chiral starting material or chiral transition states and thus it could be categorized as an area of supramolecular catalysis, but supramolecular catalysis however does not necessarily have to involve asymmetric reaction. As there is another Wikipedia article already written about small molecule asymmetric catalysts, this article focuses primarily on large catalytic host molecules. Non-discrete and structurally poorly defined system such as micelle and dendrimers are not included.