Chapter 7- Components of Optical Instruments
... For the ultraviolet re-ion, the most common source is the deuterium lamp. High-pressure, air-filled arc lamps containing argon, xenon, or mercury serve when a particularly intense source is required. For the visible region of the spectra, the tungsten filament lamp is used almost universally. The co ...
... For the ultraviolet re-ion, the most common source is the deuterium lamp. High-pressure, air-filled arc lamps containing argon, xenon, or mercury serve when a particularly intense source is required. For the visible region of the spectra, the tungsten filament lamp is used almost universally. The co ...
Lecture 23
... determined by the signal. This is usually done using some kind of feedback. Lasers work according to the same sort of idea. The idea is to use atoms or other quantum systems as a kind of circuit element. They are objects that can respond at optical frequencies, (which an LC circuit cannot!) The feed ...
... determined by the signal. This is usually done using some kind of feedback. Lasers work according to the same sort of idea. The idea is to use atoms or other quantum systems as a kind of circuit element. They are objects that can respond at optical frequencies, (which an LC circuit cannot!) The feed ...
Match the definit
... B. A particle of visible light or other electromagnetic radiation C. A fundamental substance that cannot be broken down into a simpler chemical substance D. The study and analysis of spectra E. A wave consisting of alternating electric and magnetic energy F. The theory that electromagnetic radiation ...
... B. A particle of visible light or other electromagnetic radiation C. A fundamental substance that cannot be broken down into a simpler chemical substance D. The study and analysis of spectra E. A wave consisting of alternating electric and magnetic energy F. The theory that electromagnetic radiation ...
I. Physical Biochemistry Protein Interactions Ultraviolet(UV
... is a distance-dependent interaction between the electronic excited states of two dye molecules in which excitation is transferred from a donor molecule to an acceptor molecule without emission of a photon. ...
... is a distance-dependent interaction between the electronic excited states of two dye molecules in which excitation is transferred from a donor molecule to an acceptor molecule without emission of a photon. ...
Problem Set 1 (due 2/21/06)
... modes of water are relatively small, and aqueous samples may be analyzed (water absorbs strongly in the IR). This allows analysis of a wide range of chemical and biological samples (even in vivo). Raman measures vibrational mode energies, but uses a visible wavelength light source. Optics and detect ...
... modes of water are relatively small, and aqueous samples may be analyzed (water absorbs strongly in the IR). This allows analysis of a wide range of chemical and biological samples (even in vivo). Raman measures vibrational mode energies, but uses a visible wavelength light source. Optics and detect ...
the atomic theory
... Elements are made of tiny particles called atoms. All atoms of a given element are identical. The atoms of a given element are different from those of any other element; the atoms of different elements can be distinguished from one another by their respective relative weights. Atoms of one element c ...
... Elements are made of tiny particles called atoms. All atoms of a given element are identical. The atoms of a given element are different from those of any other element; the atoms of different elements can be distinguished from one another by their respective relative weights. Atoms of one element c ...
Spectra and Atomic Structure
... Spectral Lines Kirchhoff’s laws: 1. Luminous solid, liquid, or dense gas produces continuous spectrum 2. Low-density hot gas produces emission spectrum 3. Continuous spectrum incident on cool, thin gas produces absorption spectrum ...
... Spectral Lines Kirchhoff’s laws: 1. Luminous solid, liquid, or dense gas produces continuous spectrum 2. Low-density hot gas produces emission spectrum 3. Continuous spectrum incident on cool, thin gas produces absorption spectrum ...
1.3.6 Electromagnetic radiation Name Symbol Definition SI
... power α/γl, or the molar optical rotatory power α/cl, where γ is the mass concentration, c is the amount (of substance) concentration, and l is the path length. Most tabulations give the specific optical rotatory power, denoted [α]. The wavelength of light used λ (frequently the sodium D line) and t ...
... power α/γl, or the molar optical rotatory power α/cl, where γ is the mass concentration, c is the amount (of substance) concentration, and l is the path length. Most tabulations give the specific optical rotatory power, denoted [α]. The wavelength of light used λ (frequently the sodium D line) and t ...
Introduction to Spectroscopic Methods
... The term electromagnetic radiation, is derived from the characteristic electric and magnetic properties common to all forms of this wave-like energy, as manifested by the generation of both electrical and magnetic oscillating fields as the waves propagate through space. Electromagnetic radiation is ...
... The term electromagnetic radiation, is derived from the characteristic electric and magnetic properties common to all forms of this wave-like energy, as manifested by the generation of both electrical and magnetic oscillating fields as the waves propagate through space. Electromagnetic radiation is ...
Global Doppler frequency shift detection with near-resonant interferometry A. Landolt
... The proposed method is inherently a single camera technique without image alignment and camera calibration issues. Because only the position of interference fringes needs to be determined in the post processing step, image intensity noise affects accuracy only in a minor way. The measurement range i ...
... The proposed method is inherently a single camera technique without image alignment and camera calibration issues. Because only the position of interference fringes needs to be determined in the post processing step, image intensity noise affects accuracy only in a minor way. The measurement range i ...
CHEM 305 Absorption of light: Beer
... Absorption of light: Beer-Lambert Law Up to this point, we have learned how electromagnetic waves are generated, how molecules can scatter light (and how we can determine molecular weight from the amount of scattering, using a Zimm plot) and how helical molecules interact with circularly polarized l ...
... Absorption of light: Beer-Lambert Law Up to this point, we have learned how electromagnetic waves are generated, how molecules can scatter light (and how we can determine molecular weight from the amount of scattering, using a Zimm plot) and how helical molecules interact with circularly polarized l ...
docx - Pdx
... “electrical pumping”, or stimulation by energetic electrons, to excite the atoms. Go to the One Atom (Absorption and Emission) tab. This set-up will give you a general idea of the electron energy levels of an atom. Increase the intensity of the lamp using the Lamp Control slide button. Vary the ligh ...
... “electrical pumping”, or stimulation by energetic electrons, to excite the atoms. Go to the One Atom (Absorption and Emission) tab. This set-up will give you a general idea of the electron energy levels of an atom. Increase the intensity of the lamp using the Lamp Control slide button. Vary the ligh ...
Electromagnetic radiation
... The qualitative analysis is the position (energy, frequency, length of wave, wave‘s number) of maximums (lines) in the electromagnetic spectrum. ...
... The qualitative analysis is the position (energy, frequency, length of wave, wave‘s number) of maximums (lines) in the electromagnetic spectrum. ...
Biomolecular and cellular research devices.
... Raman spectrum of giant chromosomes of a midge (Chironomus). At selected wave number values it is possible to run Raman microscopy. Excited by 647.1 nm laser light. ...
... Raman spectrum of giant chromosomes of a midge (Chironomus). At selected wave number values it is possible to run Raman microscopy. Excited by 647.1 nm laser light. ...
Optical Measurements
... • Atomic absorption measurements • Light scattering measurements: Raman Scattering, Dynamic light scattering ...
... • Atomic absorption measurements • Light scattering measurements: Raman Scattering, Dynamic light scattering ...
File
... thorium and cerium held together by a binding material). The radiation is divided into two beams, one of which passes through the sample while the other function as a reference beam. The reference and the sample beams are then passed alternately into a monochromator at very short intervals by means ...
... thorium and cerium held together by a binding material). The radiation is divided into two beams, one of which passes through the sample while the other function as a reference beam. The reference and the sample beams are then passed alternately into a monochromator at very short intervals by means ...
BJ - Faculty Web Pages
... Ignore isotope effects and exact wavenumbers above, but sketch should be close to this. (b) Predict the wavenumbers of rotational Raman transitions for J = 0, 1, 2, 3 for 14N2, for which B = 1.99 cm 1, when it is exposed to monochromatic 336.732 nm laser radiation. Stokes: 29685.27, 29677.31, 29669. ...
... Ignore isotope effects and exact wavenumbers above, but sketch should be close to this. (b) Predict the wavenumbers of rotational Raman transitions for J = 0, 1, 2, 3 for 14N2, for which B = 1.99 cm 1, when it is exposed to monochromatic 336.732 nm laser radiation. Stokes: 29685.27, 29677.31, 29669. ...
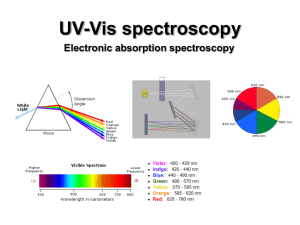
UV-Vis (electronic) spectroscopy
... electronic excitation (10-16 s). Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
... electronic excitation (10-16 s). Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
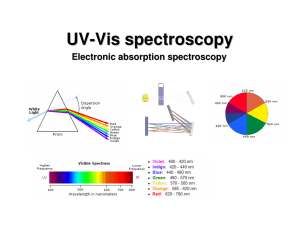
UV-Vis spectroscopy
... electronic excitation (10-16 s). Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
... electronic excitation (10-16 s). Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
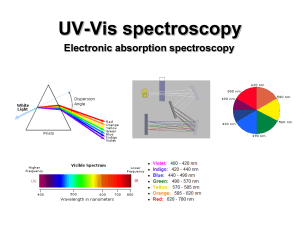
UV-Vis (electronic) spectroscopy
... electronic excitation (10-16 s). Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
... electronic excitation (10-16 s). Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
Instrumental Methods of Analysis
... Dispersive instruments: have been the traditional instrument design for IRs; high resolution is possible, FT-IR is essentially the same in modern instruments. The FT-IR has a much higher sensitivity which is very important due to the low signal levels in the IR region. This extra sensitivity makes i ...
... Dispersive instruments: have been the traditional instrument design for IRs; high resolution is possible, FT-IR is essentially the same in modern instruments. The FT-IR has a much higher sensitivity which is very important due to the low signal levels in the IR region. This extra sensitivity makes i ...
Paper
... nitrogen of a known water quantity and pumped down to the Doppler region. A high concentration cell was filled with 100% RH air and pumped down to the same pressure. A computer model was used to overlay the data and extract a concentration measurement. Figure 3 shows the profile obtained at the firs ...
... nitrogen of a known water quantity and pumped down to the Doppler region. A high concentration cell was filled with 100% RH air and pumped down to the same pressure. A computer model was used to overlay the data and extract a concentration measurement. Figure 3 shows the profile obtained at the firs ...
lecture #7 ppt
... Intensity obtained by ,multiplying by c is 3x10-6 dw W/m2 dw for an ordinary spectroscopic light source is ~1011 Hz The intensity required to equalize spontaneous and stimulated emission rates is ~105W/m2 ...
... Intensity obtained by ,multiplying by c is 3x10-6 dw W/m2 dw for an ordinary spectroscopic light source is ~1011 Hz The intensity required to equalize spontaneous and stimulated emission rates is ~105W/m2 ...
Atomic absorption spectroscopy

Atomic absorption spectroscopy (AAS) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state.In analytical chemistry the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. AAS can be used to determine over 70 different elements in solution or directly in solid samples used in pharmacology, biophysics and toxicology research.Atomic absorption spectroscopy was first used as an analytical technique, and the underlying principles were established in the second half of the 19th century by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, both professors at the University of Heidelberg, Germany.The modern form of AAS was largely developed during the 1950s by a team of Australian chemists. They were led by Sir Alan Walsh at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Division of Chemical Physics, in Melbourne, Australia.Atomic absorption spectrometry has many uses in different areas of chemistry such as: Clinical analysis: Analyzing metals in biological fluids and tissues such as whole blood, plasma, urine, saliva, brain tissue, liver, muscle tissue, semen Pharmaceuticals: In some pharmaceutical manufacturing processes, minute quantities of a catalyst that remain in the final drug product Water analysis: Analyzing water for its metal content.↑ ↑ ↑