Laser - nptel
... 2.5.2Collision Broadening: Collision of atoms and molecules among themselves interrupts a radiative transition. If an atom which is emitting a radiation suddenly collides with another atom, the process of radiation is interrupted. the radiating atom starts its motion after such a collision with a co ...
... 2.5.2Collision Broadening: Collision of atoms and molecules among themselves interrupts a radiative transition. If an atom which is emitting a radiation suddenly collides with another atom, the process of radiation is interrupted. the radiating atom starts its motion after such a collision with a co ...
slides introducing IR/Raman of proteins
... Magnetic Resonance—different course • Long wavelength radiowaves are of low energy that is sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). Nuclei interact weakly so spectral transitions between single, well defined energy levels are very sharp and well resolved. NMR is a vital tec ...
... Magnetic Resonance—different course • Long wavelength radiowaves are of low energy that is sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). Nuclei interact weakly so spectral transitions between single, well defined energy levels are very sharp and well resolved. NMR is a vital tec ...
The role of chemical reactions in the laser destruction of transparent
... In addition, we shall assume that the average number density n of inclusions is sufficiently small for the temperature field near each of them to be determined by the inclusion itself (this is valid if &V2IS << 1, where X is the mean size of a n inclusion and V is the focal volumec71). In this case ...
... In addition, we shall assume that the average number density n of inclusions is sufficiently small for the temperature field near each of them to be determined by the inclusion itself (this is valid if &V2IS << 1, where X is the mean size of a n inclusion and V is the focal volumec71). In this case ...
Practice Test #1
... The average velocity of the gas particles is directly proportional to the pressure. Gas particles are very small compared with the average distance between particlesGas particles collide with the walls of their container and in doing so give rise to pressure. Gasesare made up of tiny particles in co ...
... The average velocity of the gas particles is directly proportional to the pressure. Gas particles are very small compared with the average distance between particlesGas particles collide with the walls of their container and in doing so give rise to pressure. Gasesare made up of tiny particles in co ...
Chapter 20 Molecular Mass Spectrometry
... Advantages and Disadvantages of El Sources Electron–impact sources are convenient to use because of good sensitivities. The extensive fragmentation and consequent large number of peaks is also an advantage because it often makes unambiguous identification of analytes possible. This fragmentation c ...
... Advantages and Disadvantages of El Sources Electron–impact sources are convenient to use because of good sensitivities. The extensive fragmentation and consequent large number of peaks is also an advantage because it often makes unambiguous identification of analytes possible. This fragmentation c ...
Chem 4631 - UNT Chemistry
... Produced when solids are heated to incandescence. The thermal radiation produced is called blackbody radiation. This radiation is characteristic of the temperature of the emitting surface. ...
... Produced when solids are heated to incandescence. The thermal radiation produced is called blackbody radiation. This radiation is characteristic of the temperature of the emitting surface. ...
Optical properties of silicon at low temperatures
... • for p-polarised light there is no reflection on a surface if the angle of incident is the brewster angle • so all light can be used for the measurement without losses because of reflected light • scattered light causes problems inside a cryostat ...
... • for p-polarised light there is no reflection on a surface if the angle of incident is the brewster angle • so all light can be used for the measurement without losses because of reflected light • scattered light causes problems inside a cryostat ...
Spectroscopic methods for biology and medicine
... of shooting particles with well defined properties at the sample and analyzing particles which are emitted by the sample as indicated in Fig. 1.4. As a result, the measurement is due to the properties of the sample, the properties of the probing particle, and the physical laws governing the interact ...
... of shooting particles with well defined properties at the sample and analyzing particles which are emitted by the sample as indicated in Fig. 1.4. As a result, the measurement is due to the properties of the sample, the properties of the probing particle, and the physical laws governing the interact ...
nλ = dsinθ
... the atoms can be excited to higher energy levels within the atoms; when they return to their original levels electromagnetic radiation is emitted. Some of this radiation may be in a wavelength region that is visible to the human eye. In this experiment mercury is placed in an electric discharge tube ...
... the atoms can be excited to higher energy levels within the atoms; when they return to their original levels electromagnetic radiation is emitted. Some of this radiation may be in a wavelength region that is visible to the human eye. In this experiment mercury is placed in an electric discharge tube ...
laser2-broadening
... spectroscopy. Narrow lines are highly desirable for both absorption and emission because they reduce the possibility of interference due to overlapping spectra. The line width ½ of an atomic absorption or emission line is defined as its width in wavelength units when measured at one half the ...
... spectroscopy. Narrow lines are highly desirable for both absorption and emission because they reduce the possibility of interference due to overlapping spectra. The line width ½ of an atomic absorption or emission line is defined as its width in wavelength units when measured at one half the ...
17588_lecture10-11_11795_laser-and-its-applications2
... incident photon of energy h =E2-E1 passes by an atom in an excited state E2, it stimulates the atom to drop or decay to the lower state E1. In this process, the atom releases a photon of the same energy, direction, phase and polarization as that of the photon passing by, the net effect is two ident ...
... incident photon of energy h =E2-E1 passes by an atom in an excited state E2, it stimulates the atom to drop or decay to the lower state E1. In this process, the atom releases a photon of the same energy, direction, phase and polarization as that of the photon passing by, the net effect is two ident ...
Raman spectroscopy of nanostructures and single molecules
... dedicated sources of synchrotron light make possible to improve detection limits for trace elements in several orders of magnitude. Besides, it permits to implement spectrochemical analysis with spatial resolution on the micrometer scale. As could be expected, this kind of experiment using conventio ...
... dedicated sources of synchrotron light make possible to improve detection limits for trace elements in several orders of magnitude. Besides, it permits to implement spectrochemical analysis with spatial resolution on the micrometer scale. As could be expected, this kind of experiment using conventio ...
Spectroscopy of Atoms and Molecules
... In this laboratory exercise, we will probe the behavior of electrons within atoms using Emission and Absorbance Spectroscopy. We will first examine the photons emitted from excited atoms of various salts. Then we will observe the line spectrum of excited Hydrogen atoms and excited Helium atoms. Fina ...
... In this laboratory exercise, we will probe the behavior of electrons within atoms using Emission and Absorbance Spectroscopy. We will first examine the photons emitted from excited atoms of various salts. Then we will observe the line spectrum of excited Hydrogen atoms and excited Helium atoms. Fina ...
High Resolution Laser Spectroscopy in Rubidium
... will also include a discussion of doppler broadening and crossover resonance. We have devoted and entire section to saturation absorption spectroscopy, and it will be discussed in the section aptly named. The apparatus section will include a description of the apparatus which includes various optica ...
... will also include a discussion of doppler broadening and crossover resonance. We have devoted and entire section to saturation absorption spectroscopy, and it will be discussed in the section aptly named. The apparatus section will include a description of the apparatus which includes various optica ...
Chapter 1: The Nature of Analytical Chemistry
... solve practical problems. • Analytical chemistry is applied in all areas of science, industry, and medicine. • Chemistry: The Central Science; all sub-disciplines rely on analytical chemistry to function. ...
... solve practical problems. • Analytical chemistry is applied in all areas of science, industry, and medicine. • Chemistry: The Central Science; all sub-disciplines rely on analytical chemistry to function. ...
EXPERIMENTAL TECHNIQUES
... and all M lines. This technique is highly sensitive to the detection of low elements such as Be, B, C, N, O and F, but the measurement error is high. Quantitative analysis requires making corrections to the raw data such as deadtime, background and instrumental drift, before making the matrix correc ...
... and all M lines. This technique is highly sensitive to the detection of low elements such as Be, B, C, N, O and F, but the measurement error is high. Quantitative analysis requires making corrections to the raw data such as deadtime, background and instrumental drift, before making the matrix correc ...
... The combination explains free electrons with high absorption (R near zero) for low frequencies ( o 0 ) for the IR region of the light spectrum. The bound electrons, oscillator explain the absorption bands. Insulators and semiconductors are explained by the harmonic oscillator of bound electrons. ...
No Slide Title
... laser beam and they scatter the laser light. The scattered light is measured at right angles to the laser beam by a photodiode detector. ...
... laser beam and they scatter the laser light. The scattered light is measured at right angles to the laser beam by a photodiode detector. ...
Components of Optical Instruments, Cont…
... is to be dispersed, a quartz prism, rather than a glass, prism should be used. - Quartz serves well in both UV and Vis. - It should also be appreciated that the dispersion of a prism is nonlinear since it is ...
... is to be dispersed, a quartz prism, rather than a glass, prism should be used. - Quartz serves well in both UV and Vis. - It should also be appreciated that the dispersion of a prism is nonlinear since it is ...
CHAPTER 2: Experimental
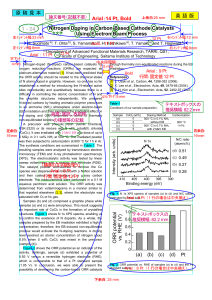
... transferred into a two-necked RB flask. An aqueous solution (3 ml) of ammonium dihydrogen phosphate (0.3 g) was added with stirring. The solution was heated at different temperatures viz. 75, 100, 125 and 185°C for two hours. The precipitate obtained was washed with methanol and acetone to remove u ...
... transferred into a two-necked RB flask. An aqueous solution (3 ml) of ammonium dihydrogen phosphate (0.3 g) was added with stirring. The solution was heated at different temperatures viz. 75, 100, 125 and 185°C for two hours. The precipitate obtained was washed with methanol and acetone to remove u ...
2011 Research Poster
... the optical trap, we diffract atoms by passing a moving standing wave through the condensate. The standing wave acts as a diffraction grating that diffracts atoms, giving them a momentum kick as first or higher order diffractions of the condensate. We split the beam into two parts by the polarizing ...
... the optical trap, we diffract atoms by passing a moving standing wave through the condensate. The standing wave acts as a diffraction grating that diffracts atoms, giving them a momentum kick as first or higher order diffractions of the condensate. We split the beam into two parts by the polarizing ...
Infrared Spectroscopy_03
... • Long pathlength (10 cm) cells – used to study dilute (few molecules) or weakly absorbing samples. • Multipass cells – more compact and efficient instead of long-pathlength cells. Mirrors are used so that the beam makes several passes through the sample before exiting the cell. (Effective pathleng ...
... • Long pathlength (10 cm) cells – used to study dilute (few molecules) or weakly absorbing samples. • Multipass cells – more compact and efficient instead of long-pathlength cells. Mirrors are used so that the beam makes several passes through the sample before exiting the cell. (Effective pathleng ...
Document
... • Long pathlength (10 cm) cells – used to study dilute (few molecules) or weakly absorbing samples. • Multipass cells – more compact and efficient instead of long-pathlength cells. Mirrors are used so that the beam makes several passes through the sample before exiting the cell. (Effective pathleng ...
... • Long pathlength (10 cm) cells – used to study dilute (few molecules) or weakly absorbing samples. • Multipass cells – more compact and efficient instead of long-pathlength cells. Mirrors are used so that the beam makes several passes through the sample before exiting the cell. (Effective pathleng ...
Effects of antioxidants for the degradation of flame
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
Atomic absorption spectroscopy

Atomic absorption spectroscopy (AAS) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state.In analytical chemistry the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. AAS can be used to determine over 70 different elements in solution or directly in solid samples used in pharmacology, biophysics and toxicology research.Atomic absorption spectroscopy was first used as an analytical technique, and the underlying principles were established in the second half of the 19th century by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, both professors at the University of Heidelberg, Germany.The modern form of AAS was largely developed during the 1950s by a team of Australian chemists. They were led by Sir Alan Walsh at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Division of Chemical Physics, in Melbourne, Australia.Atomic absorption spectrometry has many uses in different areas of chemistry such as: Clinical analysis: Analyzing metals in biological fluids and tissues such as whole blood, plasma, urine, saliva, brain tissue, liver, muscle tissue, semen Pharmaceuticals: In some pharmaceutical manufacturing processes, minute quantities of a catalyst that remain in the final drug product Water analysis: Analyzing water for its metal content.↑ ↑ ↑