FTIR Spectrometer - Pat Arnott Web Site
... Consequence: The less IR radiation escapes to space when the atmosphere has 800 ppm CO2 because the atmosphere is less transparent to IR emitted by the Earth’s surface. The Earth’s surface temperature must increase to again balance the outgoing IR with the incoming solar radiation. ...
... Consequence: The less IR radiation escapes to space when the atmosphere has 800 ppm CO2 because the atmosphere is less transparent to IR emitted by the Earth’s surface. The Earth’s surface temperature must increase to again balance the outgoing IR with the incoming solar radiation. ...
FTIR Spectrometer
... Consequence: The less IR radiation escapes to space when the atmosphere has 800 ppm CO2 because the atmosphere is less transparent to IR emitted by the Earth’s surface. The Earth’s surface temperature must increase to again balance the outgoing IR with the incoming solar radiation. ...
... Consequence: The less IR radiation escapes to space when the atmosphere has 800 ppm CO2 because the atmosphere is less transparent to IR emitted by the Earth’s surface. The Earth’s surface temperature must increase to again balance the outgoing IR with the incoming solar radiation. ...
What is remote sensing?
... Sensors are the devices for detecting the photons. The critical part of the sensors is the detectors which works based on photoelectric effect. That is, There will be an emmision of negative particles (electrons) when negatively charged plate is subject to a beam of photons. The electrons then can ...
... Sensors are the devices for detecting the photons. The critical part of the sensors is the detectors which works based on photoelectric effect. That is, There will be an emmision of negative particles (electrons) when negatively charged plate is subject to a beam of photons. The electrons then can ...
METO 621
... • To get enough energy to break up a molecule (dissociation) the wavelength must be in or below the ultraviolet. Thus dissociation typically occurs as the result of electronic transitions • Small, light chemical species generally have electronic transitions at wavelengths shorter than those for more ...
... • To get enough energy to break up a molecule (dissociation) the wavelength must be in or below the ultraviolet. Thus dissociation typically occurs as the result of electronic transitions • Small, light chemical species generally have electronic transitions at wavelengths shorter than those for more ...
Info Note 804: UV-VIS Nomenclature and Units
... A spectrophotometer can be either single beam or double beam. In a single beam instrument, all of the light passes through the sample cell. Io must be measured by removing the sample. This was the earliest design, but is still in common use in both teaching and industrial labs. Samples for UV/Vis sp ...
... A spectrophotometer can be either single beam or double beam. In a single beam instrument, all of the light passes through the sample cell. Io must be measured by removing the sample. This was the earliest design, but is still in common use in both teaching and industrial labs. Samples for UV/Vis sp ...
Document
... overcome binding energy and thus be released. In addition, radiation offered released electrons enough kinetic energy to transfer to the anode surface and overcome repulsion forces with the negative anode. If the energy of the incident beam was calculated per surface area of an electron, this energy ...
... overcome binding energy and thus be released. In addition, radiation offered released electrons enough kinetic energy to transfer to the anode surface and overcome repulsion forces with the negative anode. If the energy of the incident beam was calculated per surface area of an electron, this energy ...
Chapter 2 Classical propagation
... refractive index and the absorption coefficient are not independent parameters but are related to each other. If we invoke the law of causality (that an effect may not precede its cause) and apply complex number analysis, we can derive general relationships between the real and imaginary parts of th ...
... refractive index and the absorption coefficient are not independent parameters but are related to each other. If we invoke the law of causality (that an effect may not precede its cause) and apply complex number analysis, we can derive general relationships between the real and imaginary parts of th ...
Synthesis of a New Structure B2H4 from B2H6 Highly Selective
... branching ratios were drawn as in Fig. 2. The products with m/z = 28 u have larger branching ratios following 1s → π* transitions at the C, N, and O K-edges. The branching ratio was enhanced from 20 % to 35 % at N and O K-edges, so confirming the product from a specific dissociation. Tracing the mec ...
... branching ratios were drawn as in Fig. 2. The products with m/z = 28 u have larger branching ratios following 1s → π* transitions at the C, N, and O K-edges. The branching ratio was enhanced from 20 % to 35 % at N and O K-edges, so confirming the product from a specific dissociation. Tracing the mec ...
Chem 30A Fa_06 FE Review
... 160 Ci, what would be its activity after 24 days? How many days does it take for the activity to decrease to 5 Ci? (Answer: 20 Ci; 40 days) ...
... 160 Ci, what would be its activity after 24 days? How many days does it take for the activity to decrease to 5 Ci? (Answer: 20 Ci; 40 days) ...
Modified copy of Flame Tests 2013
... While Rutherford's experimentation and model helped to explain the inner structure of the atom, it did very little to explain the chemical behavior and periodicity of the elements. The next clue toward understanding the nature of atoms was to come from a very different study of chemistry-spectroscop ...
... While Rutherford's experimentation and model helped to explain the inner structure of the atom, it did very little to explain the chemical behavior and periodicity of the elements. The next clue toward understanding the nature of atoms was to come from a very different study of chemistry-spectroscop ...
FTIR Spectrometer - Pat Arnott Web Site
... Consequence: The less IR radiation escapes to space when the atmosphere has 800 ppm CO2 because the atmosphere is less transparent to IR emitted by the Earth’s surface. The Earth’s surface temperature must increase to again balance the outgoing IR with the incoming solar radiation. ...
... Consequence: The less IR radiation escapes to space when the atmosphere has 800 ppm CO2 because the atmosphere is less transparent to IR emitted by the Earth’s surface. The Earth’s surface temperature must increase to again balance the outgoing IR with the incoming solar radiation. ...
10.3.2.1.1 Spectral apparatus An optical arrangement or an
... arrangement or when the focal planes are different in the two dimensions, astigmatic. When the radiation passes through the same optical components before and after being dispersed, the spectral system is autocollimative. Spectral separation or isolation of optical radiation may be achieved by using ...
... arrangement or when the focal planes are different in the two dimensions, astigmatic. When the radiation passes through the same optical components before and after being dispersed, the spectral system is autocollimative. Spectral separation or isolation of optical radiation may be achieved by using ...
Atomic Emission Spectrometry - San Diego Unified School District
... emitting a photon of light. Photons are particles with energy but no mass. Their energy is directly proportional to the frequency of the light (remember: E = hf). The photons emitted precisely match the quantum energy difference between the excited state and the ground state. For different elements ...
... emitting a photon of light. Photons are particles with energy but no mass. Their energy is directly proportional to the frequency of the light (remember: E = hf). The photons emitted precisely match the quantum energy difference between the excited state and the ground state. For different elements ...
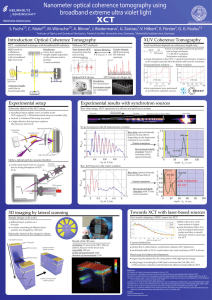
Nanometer optical coherence tomography using
... photon flux 3 orders below synchrotron radiation (10¹¹ photons/s) small bandwidth of 35 eV would reduce the axial resolution of XCT to 40 nm ...
... photon flux 3 orders below synchrotron radiation (10¹¹ photons/s) small bandwidth of 35 eV would reduce the axial resolution of XCT to 40 nm ...
Atomic_spectra
... Fraunhofer also completed an important theoretical work on diffraction and established the laws of diffraction. One important innovation that Fraunhofer made was to place a diffraction slit in front of the objective of a measuring telescope in order to study the solar spectrum. He later made and use ...
... Fraunhofer also completed an important theoretical work on diffraction and established the laws of diffraction. One important innovation that Fraunhofer made was to place a diffraction slit in front of the objective of a measuring telescope in order to study the solar spectrum. He later made and use ...
Absorption 1
... The sun in H–α (left, NASA). Details close to a sunspot (SST, O. Engvold et al.) ...
... The sun in H–α (left, NASA). Details close to a sunspot (SST, O. Engvold et al.) ...
Introduction to the principles of Atomic Spectroscopy
... In molecular spectroscopy, low-energy radiation (IR, VIS, UV) causes molecules to vibrate/rotate or outer electrons to transit from low to high energy states ...
... In molecular spectroscopy, low-energy radiation (IR, VIS, UV) causes molecules to vibrate/rotate or outer electrons to transit from low to high energy states ...
excited states
... • Pf = Po×F×2.303×e×b×C or Pf = K×C or a linear response in the fluorescence intensity with concentration will be observed as long as the A < ...
... • Pf = Po×F×2.303×e×b×C or Pf = K×C or a linear response in the fluorescence intensity with concentration will be observed as long as the A < ...
Phys 282 EXP 8
... To study the time dependence of radioactive decay. To find half-life time for different materials. APPARATUS: Geiger-Mueller counter with computer interface, radioactive sources. ...
... To study the time dependence of radioactive decay. To find half-life time for different materials. APPARATUS: Geiger-Mueller counter with computer interface, radioactive sources. ...
experiment 8 radioactive decay of nuclei
... Indium metal (49In, atomic number of 49) as found on the surface of the earth is 95.72 % mass 115 and 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where th ...
... Indium metal (49In, atomic number of 49) as found on the surface of the earth is 95.72 % mass 115 and 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where th ...
Structural, electronic and optical properties of TiO2 nanoparticles
... − TiO2 pigments are widely used in the industry: whiteness, opacity − Nano-TiO2: Plastics, coatings, cosmetics − Particle size and shape distribution important for applications − These distributions can be solved by measuring the turbidity spectrum of a dilute solution: A nontrivial inverse problem ...
... − TiO2 pigments are widely used in the industry: whiteness, opacity − Nano-TiO2: Plastics, coatings, cosmetics − Particle size and shape distribution important for applications − These distributions can be solved by measuring the turbidity spectrum of a dilute solution: A nontrivial inverse problem ...
1- Light Variation Study of the Eclipsing System (W -... Layth T. H. Kadouri Talib H. Kadouri
... variable and its components are moving to form an open eight figure orbit () . The results of this research demonstrate that there is no evidence supports this idea. The light curve is also analyzed on the base that the system is an eclipsing binary. Photometric physical and geometrical parameters, ...
... variable and its components are moving to form an open eight figure orbit () . The results of this research demonstrate that there is no evidence supports this idea. The light curve is also analyzed on the base that the system is an eclipsing binary. Photometric physical and geometrical parameters, ...
Gr 10 Review sheet chemistry
... 1. Change of________________ 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
... 1. Change of________________ 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
Atomic absorption spectroscopy

Atomic absorption spectroscopy (AAS) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state.In analytical chemistry the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. AAS can be used to determine over 70 different elements in solution or directly in solid samples used in pharmacology, biophysics and toxicology research.Atomic absorption spectroscopy was first used as an analytical technique, and the underlying principles were established in the second half of the 19th century by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, both professors at the University of Heidelberg, Germany.The modern form of AAS was largely developed during the 1950s by a team of Australian chemists. They were led by Sir Alan Walsh at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Division of Chemical Physics, in Melbourne, Australia.Atomic absorption spectrometry has many uses in different areas of chemistry such as: Clinical analysis: Analyzing metals in biological fluids and tissues such as whole blood, plasma, urine, saliva, brain tissue, liver, muscle tissue, semen Pharmaceuticals: In some pharmaceutical manufacturing processes, minute quantities of a catalyst that remain in the final drug product Water analysis: Analyzing water for its metal content.↑ ↑ ↑