Physical and Chemical Tests
... The actual energy difference is small. At 300 MHz, the energy difference for a proton is about 3 x 10-5 kcal mol-1. Because the energy difference is so small and the equilibrium between the two states is so fast, the numbers of nuclei in the two states are nearly equal, however, a slight excess will ...
... The actual energy difference is small. At 300 MHz, the energy difference for a proton is about 3 x 10-5 kcal mol-1. Because the energy difference is so small and the equilibrium between the two states is so fast, the numbers of nuclei in the two states are nearly equal, however, a slight excess will ...
Chapter8_notes
... 2) Atomic absorption spectra • The spectra result from the atomized sample absorbing photons of radiation of the appropriate energy (wavelength). • Resonance absorption lines are the most prevalent observed since few of the atoms are in the excited state. (e.g. 3s 4p in Na°) 3) Atomic fluorescenc ...
... 2) Atomic absorption spectra • The spectra result from the atomized sample absorbing photons of radiation of the appropriate energy (wavelength). • Resonance absorption lines are the most prevalent observed since few of the atoms are in the excited state. (e.g. 3s 4p in Na°) 3) Atomic fluorescenc ...
lecture1
... O at 5.9 µm. Acids (-C=O-OH) and esters (-C=O-OR) absorb at lower wavelength while amides (-C=O-NH2) absorb at longer wavelength with two peaks, hence discrimination is possible. These three regions are the “functional group” region. The fourth region is the “fingerprint” region. This is the single ...
... O at 5.9 µm. Acids (-C=O-OH) and esters (-C=O-OR) absorb at lower wavelength while amides (-C=O-NH2) absorb at longer wavelength with two peaks, hence discrimination is possible. These three regions are the “functional group” region. The fourth region is the “fingerprint” region. This is the single ...
Materialanalytik Praktikum UV-VIS Absorption B507
... by the color wheel shown on Figure 3A. It can be clearly seen that the complementary colors are utterly opposite each other. For example, absorption of 420-430 nm light gives rise to yellow colored substance in the transmission mode. The UV-VIS spectral range is approximately 190 to 750 nm, as defin ...
... by the color wheel shown on Figure 3A. It can be clearly seen that the complementary colors are utterly opposite each other. For example, absorption of 420-430 nm light gives rise to yellow colored substance in the transmission mode. The UV-VIS spectral range is approximately 190 to 750 nm, as defin ...
The Spectrophotometer
... The “light control” shown in the diagram consists of a slotted piece of metal which can be moved back and forth in the light path. The bulb produces varying intensities at different wavelengths. The light control decreases high intensities by blocking some of the light with the slotted metal. The ri ...
... The “light control” shown in the diagram consists of a slotted piece of metal which can be moved back and forth in the light path. The bulb produces varying intensities at different wavelengths. The light control decreases high intensities by blocking some of the light with the slotted metal. The ri ...
METO 621
... • This energy selectivity is an outstanding characteristic of absorption. Scattering is generally much less selective and occurs over a much larger region of the spectrum. • As we shall see later, molecules have a myriad of discrete energy levels and hence have complex absorption spectra. • One reas ...
... • This energy selectivity is an outstanding characteristic of absorption. Scattering is generally much less selective and occurs over a much larger region of the spectrum. • As we shall see later, molecules have a myriad of discrete energy levels and hence have complex absorption spectra. • One reas ...
Chapter 4 Spectroscopy
... passes through a cool gas, atoms of the gas will absorb the same frequencies they emit ...
... passes through a cool gas, atoms of the gas will absorb the same frequencies they emit ...
Statistics and chemometrics for analytical chemistry
... J. N. Miller Other References Course Description: ...
... J. N. Miller Other References Course Description: ...
Absorption Measurements on PC1
... (filter holder channel), the accessory can be used also with acquisition through the right emission channel (monochromator channel). In the following, we will assume that the accessory will be utilized with the left emission channel. Please keep the left emission shutter closed. Turn the left emissi ...
... (filter holder channel), the accessory can be used also with acquisition through the right emission channel (monochromator channel). In the following, we will assume that the accessory will be utilized with the left emission channel. Please keep the left emission shutter closed. Turn the left emissi ...
4) Spectroscopies Involving Energy Exchange
... constant pH to eliminate pH related chemical deviations. ...
... constant pH to eliminate pH related chemical deviations. ...
AstronomicalSpectroscopy
... • Note that the emission extends over a range of frequencies. • The term often refers to the visible light emission spectrum, although it extends to the whole electromagnetic spectrum, from the low energy radio waves up to high energy gamma rays. ...
... • Note that the emission extends over a range of frequencies. • The term often refers to the visible light emission spectrum, although it extends to the whole electromagnetic spectrum, from the low energy radio waves up to high energy gamma rays. ...
Introduction to Spectroscopy
... Interactions with matter • Ionizing – enough energy to liberate e• Non-ionizing – in general: reflection, transmission or absorption – Absorbed radiation may be re-radiated (scattered) at the original frequency (Rayleigh scattering) or at a different frequency (Raman, Brillouin, fluorescence, etc.) ...
... Interactions with matter • Ionizing – enough energy to liberate e• Non-ionizing – in general: reflection, transmission or absorption – Absorbed radiation may be re-radiated (scattered) at the original frequency (Rayleigh scattering) or at a different frequency (Raman, Brillouin, fluorescence, etc.) ...
declaração - Sistema de Eventos
... Introduction: The change in the optical path with the heat deposited per unit volume (ds/dQ) is a parameter that measures the TL distortion induced within a given material by laser beam, and it is related with the temperature coefficient of the optical path length change by ds/dT = (1/cp)ds/dQ, in ...
... Introduction: The change in the optical path with the heat deposited per unit volume (ds/dQ) is a parameter that measures the TL distortion induced within a given material by laser beam, and it is related with the temperature coefficient of the optical path length change by ds/dT = (1/cp)ds/dQ, in ...
Introduction to Spectrochemical Methods
... and sometimes in the IR region as light, although strictly speaking the term refers only to visible radiation. • Electromagnetic radiation can be described as a wave with properties of wavelength, frequency, velocity and amplitude. ...
... and sometimes in the IR region as light, although strictly speaking the term refers only to visible radiation. • Electromagnetic radiation can be described as a wave with properties of wavelength, frequency, velocity and amplitude. ...
الشريحة 1
... An example may be a visible photometer which, in principle, can be used in the range from 340-780 nm. It may be obvious that glass windows, cells and prism will start to absorb significantly below 380 nm and thus a decrease in the incident radiant power is ...
... An example may be a visible photometer which, in principle, can be used in the range from 340-780 nm. It may be obvious that glass windows, cells and prism will start to absorb significantly below 380 nm and thus a decrease in the incident radiant power is ...
h - Pharos University in Alexandria
... sensitive (single photons) linear flat response v. within limitations stable w/ time (sensitive decreases over time, weeks to months) – fast ...
... sensitive (single photons) linear flat response v. within limitations stable w/ time (sensitive decreases over time, weeks to months) – fast ...
UV-Vis Absorption Spectroscopy
... Effect of Scattered Radiation at Wavelength Extremes of an Instrument Wavelength extremes of an instrument are dependent on type of source, detector and optical components used in the manufacture of the instrument. Outside the working range of the instrument, it is not possible to use it for accura ...
... Effect of Scattered Radiation at Wavelength Extremes of an Instrument Wavelength extremes of an instrument are dependent on type of source, detector and optical components used in the manufacture of the instrument. Outside the working range of the instrument, it is not possible to use it for accura ...
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
Fourier Transform IR Spectroscopy
... • Outwards from the centreburst the cosine waves cancel and reinforce and the amplitude of the interferogram dies off. ...
... • Outwards from the centreburst the cosine waves cancel and reinforce and the amplitude of the interferogram dies off. ...
CH915: Elemental Analysis
... But: poor sensitivity (not very efficient method, most of sample lost) ...
... But: poor sensitivity (not very efficient method, most of sample lost) ...
Medical Laboratory Instrumentation 2010
... almost any type of sample. • AAS are that no information is obtained on the chemical form of the analyte (no “speciation”) and that often only one element can be determined at a time. • This last disadvantage makes AAS of very limited use for qualitative analysis. • AAS is used almost exclusively fo ...
... almost any type of sample. • AAS are that no information is obtained on the chemical form of the analyte (no “speciation”) and that often only one element can be determined at a time. • This last disadvantage makes AAS of very limited use for qualitative analysis. • AAS is used almost exclusively fo ...
Medical Laboratory Instrumentation 2010-2011 Third Year
... almost any type of sample. • AAS are that no information is obtained on the chemical form of the analyte (no “speciation”) and that often only one element can be etermined at a time. • This last disadvantage makes AAS of very limited use for qualitative analysis. • AAS is used almost exclusively for ...
... almost any type of sample. • AAS are that no information is obtained on the chemical form of the analyte (no “speciation”) and that often only one element can be etermined at a time. • This last disadvantage makes AAS of very limited use for qualitative analysis. • AAS is used almost exclusively for ...
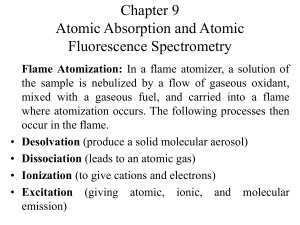
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... linearly related to the concentration of the analyte in the sample. A disadvantage of the procedure is that separate lamp source is needed for each element. ...
... linearly related to the concentration of the analyte in the sample. A disadvantage of the procedure is that separate lamp source is needed for each element. ...
Module 1 - Identifying Metals Using Atomic Emission
... Diluent—in Chemistry it is the component added to lessen concentration. Dilution—the process of lessening the concentration of a solution. Element—the basic substance formed by a specific number of atoms. Hollow Cathode Lamps—the most common radiation source in AAS. Inside the sealed lamp, filled wi ...
... Diluent—in Chemistry it is the component added to lessen concentration. Dilution—the process of lessening the concentration of a solution. Element—the basic substance formed by a specific number of atoms. Hollow Cathode Lamps—the most common radiation source in AAS. Inside the sealed lamp, filled wi ...
Atomic absorption spectroscopy

Atomic absorption spectroscopy (AAS) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state.In analytical chemistry the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. AAS can be used to determine over 70 different elements in solution or directly in solid samples used in pharmacology, biophysics and toxicology research.Atomic absorption spectroscopy was first used as an analytical technique, and the underlying principles were established in the second half of the 19th century by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, both professors at the University of Heidelberg, Germany.The modern form of AAS was largely developed during the 1950s by a team of Australian chemists. They were led by Sir Alan Walsh at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Division of Chemical Physics, in Melbourne, Australia.Atomic absorption spectrometry has many uses in different areas of chemistry such as: Clinical analysis: Analyzing metals in biological fluids and tissues such as whole blood, plasma, urine, saliva, brain tissue, liver, muscle tissue, semen Pharmaceuticals: In some pharmaceutical manufacturing processes, minute quantities of a catalyst that remain in the final drug product Water analysis: Analyzing water for its metal content.↑ ↑ ↑