Introduction into thermodynamics Thermodynamic variables
... at constant temperature and pressure. Such conditions can be maintained in a laboratory or attained in nature. Gibbs free energy is therefore of central importance in thermodynamics. It is derived from internal energy and therefore has no absolute scale. Gibbs free energy is de ned as G = U + PV − T ...
... at constant temperature and pressure. Such conditions can be maintained in a laboratory or attained in nature. Gibbs free energy is therefore of central importance in thermodynamics. It is derived from internal energy and therefore has no absolute scale. Gibbs free energy is de ned as G = U + PV − T ...
Handout 5
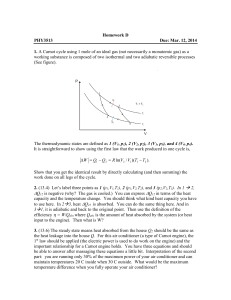
... Question: A turbine runs at 100C and has a cold sink at 20C. If the system draws 10 tons of steam which all condenses to water to deliver heat with no temperature change, then what theoretical work can be done by the turbine assuming theoretical efficiency? The heat of vaporization of water is aroun ...
... Question: A turbine runs at 100C and has a cold sink at 20C. If the system draws 10 tons of steam which all condenses to water to deliver heat with no temperature change, then what theoretical work can be done by the turbine assuming theoretical efficiency? The heat of vaporization of water is aroun ...
Thermodynamics
... of 0.010 m2. How much work can be done by a gas in the cylinder if the gas exerts a constant pressure of 7.5 x 105 Pa on the piston and moves the piston a distance of 0.040m? ...
... of 0.010 m2. How much work can be done by a gas in the cylinder if the gas exerts a constant pressure of 7.5 x 105 Pa on the piston and moves the piston a distance of 0.040m? ...
Course 3: Pressure – Volume – Temperature Relationship of Pure
... and isothermal compression coefficients of liquids are small A useful idealization known as incompressible fluid is employed in fluid mechanics for a sufficiently realistic model of liquid behavior The volume expansion and isothermal compression coefficient of the incompressible fluid are zero so it ...
... and isothermal compression coefficients of liquids are small A useful idealization known as incompressible fluid is employed in fluid mechanics for a sufficiently realistic model of liquid behavior The volume expansion and isothermal compression coefficient of the incompressible fluid are zero so it ...
chapter12_PC
... A state variable related to the Second Law of Thermodynamics, the entropy Let Qr be the energy absorbed or expelled during a reversible, constant temperature process between two equilibrium states. Then the change in entropy during any constant temperature process connecting the two equilibrium stat ...
... A state variable related to the Second Law of Thermodynamics, the entropy Let Qr be the energy absorbed or expelled during a reversible, constant temperature process between two equilibrium states. Then the change in entropy during any constant temperature process connecting the two equilibrium stat ...
ENT 211 Tutorial Week 1
... On a downhill road the potential energy of the bicyclist is being converted to kinetic energy, and thus the bicyclist picks up speed. There is no creation of energy, and thus no violation of the conservation of energy principle. ...
... On a downhill road the potential energy of the bicyclist is being converted to kinetic energy, and thus the bicyclist picks up speed. There is no creation of energy, and thus no violation of the conservation of energy principle. ...
Lecture 4 - Purdue University
... ►We defined expansion and contraction work and calculated work associated with a process in a piston cylinder device. ►We learned the potential for extension of our mechanical work knowledge metaphorically to electrical, magnetic, surface tension, torsion and other work interactions. ►We learned abo ...
... ►We defined expansion and contraction work and calculated work associated with a process in a piston cylinder device. ►We learned the potential for extension of our mechanical work knowledge metaphorically to electrical, magnetic, surface tension, torsion and other work interactions. ►We learned abo ...
Thermodynamics of ideal gases
... mechanics these degrees of freedom will also carry a kinetic energy 12 kT per particle. Molecules also possess vibrational degrees of freedom that may become excited, but we shall disregard them here. The internal energy of N particles of an ideal gas is defined to be, ...
... mechanics these degrees of freedom will also carry a kinetic energy 12 kT per particle. Molecules also possess vibrational degrees of freedom that may become excited, but we shall disregard them here. The internal energy of N particles of an ideal gas is defined to be, ...
Thermal Physics
... ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the temperature reaches its original value. See Fig. 15–22. Calculate (a) the total work done by the gas in the process, (b) the change i ...
... ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the temperature reaches its original value. See Fig. 15–22. Calculate (a) the total work done by the gas in the process, (b) the change i ...
MME 2006 Metallurgical Thermodynamics
... The isotherms for the liquid phase on the left side of the P-V and T-V diagrams are steep and closely spaced which means both volume expansion and isothermal compression coefficients of liquids are small A useful idealization known as incompressible fluid is employed in fluid mechanics for a suffici ...
... The isotherms for the liquid phase on the left side of the P-V and T-V diagrams are steep and closely spaced which means both volume expansion and isothermal compression coefficients of liquids are small A useful idealization known as incompressible fluid is employed in fluid mechanics for a suffici ...
15.1,2
... system and its surroundings. In part a, the system gains 1500 J of heat from its surroundings, and 2200 J of work is done by the system on the surroundings. In part b, the system also gains 1500 J of heat, but 2200 J of work is done on the system by the surroundings. In each case, determine the chan ...
... system and its surroundings. In part a, the system gains 1500 J of heat from its surroundings, and 2200 J of work is done by the system on the surroundings. In part b, the system also gains 1500 J of heat, but 2200 J of work is done on the system by the surroundings. In each case, determine the chan ...
HormonesCascade
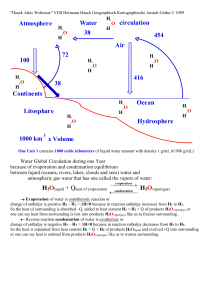
... change of enthalpy is positive H2 – H1 = ∆H>0 because in reaction enthalpy increases from H1 to H2. So the heat of surrounding is absorbed –Q, added to heat content H2 = H1 + Q of products H2Ovapor(gas) or one can say heat from surrounding is lost into products H2Ovapor(gas) like as to freezes surro ...
... change of enthalpy is positive H2 – H1 = ∆H>0 because in reaction enthalpy increases from H1 to H2. So the heat of surrounding is absorbed –Q, added to heat content H2 = H1 + Q of products H2Ovapor(gas) or one can say heat from surrounding is lost into products H2Ovapor(gas) like as to freezes surro ...
Using the “Clicker”
... A heat engine A heat engine is a device that uses heat to do work. A gasoline-powered car engine is a good example. To be useful, the engine must go through cycles, with work being done every cycle. Two temperatures are required. The higher temperature causes the system to expand, doing work, and t ...
... A heat engine A heat engine is a device that uses heat to do work. A gasoline-powered car engine is a good example. To be useful, the engine must go through cycles, with work being done every cycle. Two temperatures are required. The higher temperature causes the system to expand, doing work, and t ...
First law of thermodynamics
... surroundings are at different temperatures and the system undergoes a process, the energy transferred by non-mechanical means to or from the system is referred to as thermal energy (heat). 10.2.3 Identify the first law of thermodynamics as a statement of the principle of energy conservation. 10.2.4 ...
... surroundings are at different temperatures and the system undergoes a process, the energy transferred by non-mechanical means to or from the system is referred to as thermal energy (heat). 10.2.3 Identify the first law of thermodynamics as a statement of the principle of energy conservation. 10.2.4 ...