Episode 534 - Teaching Advanced Physics
... that were being discovered. It was possible to identify elements from the spectrum of the light they gave out when heated. It should be possible to do much the same for radioactive sources using these newer emissions. What they were beginning to realise at this time was that a good emitter like radi ...
... that were being discovered. It was possible to identify elements from the spectrum of the light they gave out when heated. It should be possible to do much the same for radioactive sources using these newer emissions. What they were beginning to realise at this time was that a good emitter like radi ...
Spectrophotometry Chapter 18
... • The electrons of an atom have different energies. • Not all energies exist, only certain allowed energy levels. • Electrons with more energy are able to get farther away from the nucleus and its + charges. • Therefore, electrons in higher energy levels spend more time farther away from the nucleus ...
... • The electrons of an atom have different energies. • Not all energies exist, only certain allowed energy levels. • Electrons with more energy are able to get farther away from the nucleus and its + charges. • Therefore, electrons in higher energy levels spend more time farther away from the nucleus ...
PYP001-121 Major-I Solution. In all the questions, choice
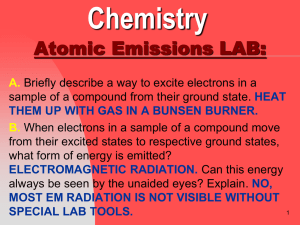
... In all the questions, choice “A” is the correct answer Q1. Which of the following statements is FALSE? A) Smoke is a compound. B) A pure substance can be either an element or compound. C) A fruit salad is a heterogeneous mixture. D) Every type of atom has a different number of protons. E) The change ...
... In all the questions, choice “A” is the correct answer Q1. Which of the following statements is FALSE? A) Smoke is a compound. B) A pure substance can be either an element or compound. C) A fruit salad is a heterogeneous mixture. D) Every type of atom has a different number of protons. E) The change ...
Problem 1 - Department of Physics and Astronomy : University of
... The medical imaging technique of positron emission tomography (PET) begins by injecting +emitting radiopharmaceuticals into a patient. The + particle is a positively charged electron called the positron. It is the electron’s anti-particle. It will travel in the body until it approaches an electron ...
... The medical imaging technique of positron emission tomography (PET) begins by injecting +emitting radiopharmaceuticals into a patient. The + particle is a positively charged electron called the positron. It is the electron’s anti-particle. It will travel in the body until it approaches an electron ...
Háskóli Íslands Raunvísindadeild,
... Spectra lines due to transitions from quantum levels n1 = 3,4, ... to n2 = 2 are named Balmer lines and appear in the visible spectral region. ...
... Spectra lines due to transitions from quantum levels n1 = 3,4, ... to n2 = 2 are named Balmer lines and appear in the visible spectral region. ...
The Light of your Life
... • Hot and cold are relative terms • You need a background continuum to have absorption lines • You can also have emission lines on a background continuum ...
... • Hot and cold are relative terms • You need a background continuum to have absorption lines • You can also have emission lines on a background continuum ...
Document
... Semiconductor detectors are found in many fields of physical research and industry ...
... Semiconductor detectors are found in many fields of physical research and industry ...
AstronomicalSpectroscopy
... • Note that the emission extends over a range of frequencies. • The term often refers to the visible light emission spectrum, although it extends to the whole electromagnetic spectrum, from the low energy radio waves up to high energy gamma rays. ...
... • Note that the emission extends over a range of frequencies. • The term often refers to the visible light emission spectrum, although it extends to the whole electromagnetic spectrum, from the low energy radio waves up to high energy gamma rays. ...
Syllabus of PHY445/515 Atomic, Molecular and Optical Physics Low
... Diode Laser Saturation Spectroscopy: Measure the Doppler broadened absorption spectrum of atomic Rb (5s-5p) using a tunable diode laser. Then, use saturation spectroscopy to measure the Doppler free spectrum which allows one to resolve the hyperfine structure of both the ground and excited states. M ...
... Diode Laser Saturation Spectroscopy: Measure the Doppler broadened absorption spectrum of atomic Rb (5s-5p) using a tunable diode laser. Then, use saturation spectroscopy to measure the Doppler free spectrum which allows one to resolve the hyperfine structure of both the ground and excited states. M ...
Red Tide Specifications
... Specify standard or SAG+. Light enters the spectrometer, passes through the SMA Connector, Slit, and Filter, and then reflects off the Collimating Mirror onto the Grating. Diffracts light from the Collimating Mirror and directs the diffracted light onto the ...
... Specify standard or SAG+. Light enters the spectrometer, passes through the SMA Connector, Slit, and Filter, and then reflects off the Collimating Mirror onto the Grating. Diffracts light from the Collimating Mirror and directs the diffracted light onto the ...
Part no: 55000-280 *(Shown with
... (1 to 15Hz, 1 to 2.7µm), flickering infra-red radiation emitted by flames during combustion. Since it responds to flickering radiation, the XP95 Dual IR Flame Detector can operate even if the lens is contaminated by a layer of oil, dust, water-vapour or ice. False alarms due to such factors as flick ...
... (1 to 15Hz, 1 to 2.7µm), flickering infra-red radiation emitted by flames during combustion. Since it responds to flickering radiation, the XP95 Dual IR Flame Detector can operate even if the lens is contaminated by a layer of oil, dust, water-vapour or ice. False alarms due to such factors as flick ...
Class25_review - Rensselaer Polytechnic Institute
... However, a “real” understanding of this was not achieved until the 1950’s. ...
... However, a “real” understanding of this was not achieved until the 1950’s. ...
supplemental problems
... a) What is the cutoff wavelength for this PMT? b) Will this PMT work in the visible portion of the spectrum? Why? Assume you are measuring 550 nm light at one point in the experiment and that 20 picoWatts of the light is incident upon the detector. The photocathode has a quantum efficiency of 22% at ...
... a) What is the cutoff wavelength for this PMT? b) Will this PMT work in the visible portion of the spectrum? Why? Assume you are measuring 550 nm light at one point in the experiment and that 20 picoWatts of the light is incident upon the detector. The photocathode has a quantum efficiency of 22% at ...
Gamma spectroscopy

Gamma-ray spectroscopy is the quantitative study of the energy spectra of gamma-ray sources, in such as the nuclear industry, geochemical investigation, and astrophysics. Most radioactive sources produce gamma rays, which are of various energies and intensities. When these emissions are detected and analyzed with a spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source, just as in optical spectroscopy, the optical spectrum is characteristic of the material contained in a sample.