nuclear test 2006
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
File
... Do Now Nuclear Chemistry 17. Which equation represents a transmutation reaction? A) B) C) D) 18. A change in the nucleus of an atom that converts the atom from one element to another element is called A) combustion C) polymerization ...
... Do Now Nuclear Chemistry 17. Which equation represents a transmutation reaction? A) B) C) D) 18. A change in the nucleus of an atom that converts the atom from one element to another element is called A) combustion C) polymerization ...
Introduction to the physics of light
... An absorption line is produced when a photon of just the right energy is absorbed by an atom, kicking an electron to a higher energy orbit. Other photons moving through the gas with the wrong energy will pass right on by the atoms in the thin gas. They make up the rest of the continuous spectrum you ...
... An absorption line is produced when a photon of just the right energy is absorbed by an atom, kicking an electron to a higher energy orbit. Other photons moving through the gas with the wrong energy will pass right on by the atoms in the thin gas. They make up the rest of the continuous spectrum you ...
Chemistry: Matter and Change
... that led to understanding radiation. • Identify alpha, beta, and gamma radiations in terms of composition and key properties. ...
... that led to understanding radiation. • Identify alpha, beta, and gamma radiations in terms of composition and key properties. ...
Candidate 2 - Elgin Academy
... attracted to the positively charged plate generating a small current. When smoke enters the chamber the smoke particles attach to the ions and reduce the current flowing. This drop in current triggers the alarm. These are very sensitive detectors. The amount of Americium 241 used in smoke detectors ...
... attracted to the positively charged plate generating a small current. When smoke enters the chamber the smoke particles attach to the ions and reduce the current flowing. This drop in current triggers the alarm. These are very sensitive detectors. The amount of Americium 241 used in smoke detectors ...
Chapter 6. Light Source and Detectors
... If more intense light falls on the photocathode, it will release more electrons but their energies, and their velocities, will remain the same. The energy of the photoelectrons depends on the frequency of the light: blue light produces more energetic photo-electrons than red light. The response o ...
... If more intense light falls on the photocathode, it will release more electrons but their energies, and their velocities, will remain the same. The energy of the photoelectrons depends on the frequency of the light: blue light produces more energetic photo-electrons than red light. The response o ...
FTIR Spectrometer - Pat Arnott Web Site
... Example Problem: Instantly Double CO2 Concentration. What is the effect on the infrared spectrum at the surface? ...
... Example Problem: Instantly Double CO2 Concentration. What is the effect on the infrared spectrum at the surface? ...
FTIR Spectrometer
... Example Problem: Instantly Double CO2 Concentration. What is the effect on the infrared spectrum at the surface? ...
... Example Problem: Instantly Double CO2 Concentration. What is the effect on the infrared spectrum at the surface? ...
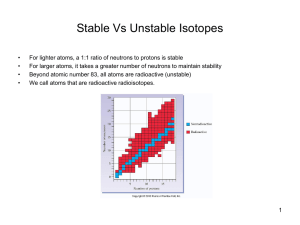
Stable Vs Unstable Isotopes
... Nuclear reactions are accompanied by tremendous energy changes as an unstable isotope spontaneously undergoes changes. ...
... Nuclear reactions are accompanied by tremendous energy changes as an unstable isotope spontaneously undergoes changes. ...
Radioactivity
... This means that they have no mass and no charge. • γ rays have a high penetrating power - it takes a thick sheet of metal such as lead, or concrete to reduce them significantly. • γ rays do not directly ionise other atoms, although they may cause atoms to emit other particles which will then cause i ...
... This means that they have no mass and no charge. • γ rays have a high penetrating power - it takes a thick sheet of metal such as lead, or concrete to reduce them significantly. • γ rays do not directly ionise other atoms, although they may cause atoms to emit other particles which will then cause i ...
Part-VI
... The maximum achievable gain, limited by breakdown, as a function of the x-ray flux for various detectors: (1) PPAC with 3mm gap; (2) MICROMEGAS; (3) PPAC with 0.6mm gap; (4) microstrip gas chamber with 1mm strip pitch; (5) microstrip gas chamber with 0.2mm strip pitch; (6) GEM; (7) microgap detector ...
... The maximum achievable gain, limited by breakdown, as a function of the x-ray flux for various detectors: (1) PPAC with 3mm gap; (2) MICROMEGAS; (3) PPAC with 0.6mm gap; (4) microstrip gas chamber with 1mm strip pitch; (5) microstrip gas chamber with 0.2mm strip pitch; (6) GEM; (7) microgap detector ...
Gamma spectroscopy

Gamma-ray spectroscopy is the quantitative study of the energy spectra of gamma-ray sources, in such as the nuclear industry, geochemical investigation, and astrophysics. Most radioactive sources produce gamma rays, which are of various energies and intensities. When these emissions are detected and analyzed with a spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source, just as in optical spectroscopy, the optical spectrum is characteristic of the material contained in a sample.