Bonding - Berkeley City College
... •The 'd+' and 'd-' symbols indicate partial positive and negative charges. •The arrow indicates the "pull" of electrons off the hydrogen and towards the more electronegative atom. ...
... •The 'd+' and 'd-' symbols indicate partial positive and negative charges. •The arrow indicates the "pull" of electrons off the hydrogen and towards the more electronegative atom. ...
Inorganic Chemistry Lesson 3
... In that case, the mass of the nail increases when it rusts. This, as well as many similar phenomena were interpreted by ordinary peoples and by scientists as an indication that matter is not necessarily conserved during chemical reactions, and this wrong belief was common among people until late XVI ...
... In that case, the mass of the nail increases when it rusts. This, as well as many similar phenomena were interpreted by ordinary peoples and by scientists as an indication that matter is not necessarily conserved during chemical reactions, and this wrong belief was common among people until late XVI ...
Chemistry Final Review 2017 1. List a set of elements
... 19. How can you distinguish between formulas represent one ionic compound and one molecular compound? 20. Which element forms an ionic compound when it reacts with lithium? 21. The bonds in BaO are best described as __. 22. Which type of bond results when one or more valence electrons are transferre ...
... 19. How can you distinguish between formulas represent one ionic compound and one molecular compound? 20. Which element forms an ionic compound when it reacts with lithium? 21. The bonds in BaO are best described as __. 22. Which type of bond results when one or more valence electrons are transferre ...
Interaction between hydrogen molecules and - FHI
... Local density of states 共LDOS兲 of metallofullerenes are presented in Fig. 2共c兲, in comparison to those of pristine fullerenes in Fig. 2共b兲, where the yellow-dotted lines representing Fermi levels EF. For large metallofullerenes La@ Cn 共n ⱖ 50兲, the La 4f, 5d, and 6s states are located above the Ferm ...
... Local density of states 共LDOS兲 of metallofullerenes are presented in Fig. 2共c兲, in comparison to those of pristine fullerenes in Fig. 2共b兲, where the yellow-dotted lines representing Fermi levels EF. For large metallofullerenes La@ Cn 共n ⱖ 50兲, the La 4f, 5d, and 6s states are located above the Ferm ...
2 KClO 3
... Wheels + Pedals + Handlebar ---» Bicycle Unbalanced: a list of ingredients & results ...
... Wheels + Pedals + Handlebar ---» Bicycle Unbalanced: a list of ingredients & results ...
PERIODIC TABLE
... 40- When (C8H16) is burned in oxygen atmosphere, we obtain (CO2) and (H2O) according to the following equation: a C8H16 + b O2 → c CO2 + d H2O In a balanced equation, the factors a, b, c, and d have the values: a- (a = 1, b = 1, c = 1, d = 1) b- (a = 1, b = 12, c = 8, d = 16) c- (a = 1, b = 12, c = ...
... 40- When (C8H16) is burned in oxygen atmosphere, we obtain (CO2) and (H2O) according to the following equation: a C8H16 + b O2 → c CO2 + d H2O In a balanced equation, the factors a, b, c, and d have the values: a- (a = 1, b = 1, c = 1, d = 1) b- (a = 1, b = 12, c = 8, d = 16) c- (a = 1, b = 12, c = ...
Chemical Equations
... • A + B ---> AB Reaction Types: Combustion •Combustion, at its most general, can mean the reaction of oxygen gas (O2) with anything. •However, we will understand combustion to mean the reaction of oxygen with a compound containing carbon and hydrogen. A common synonym for combustion is burn. •Writte ...
... • A + B ---> AB Reaction Types: Combustion •Combustion, at its most general, can mean the reaction of oxygen gas (O2) with anything. •However, we will understand combustion to mean the reaction of oxygen with a compound containing carbon and hydrogen. A common synonym for combustion is burn. •Writte ...
Notes 2 Balancing
... • Products and reactants of a reaction are made up of the same number and types of atoms. • The molecules may change but the atoms within them do not. • If you have H, O, and C are the reactant side of a reaction, you must have ___ , ___ , and ____ on the product side. • The ONLY thing we can change ...
... • Products and reactants of a reaction are made up of the same number and types of atoms. • The molecules may change but the atoms within them do not. • If you have H, O, and C are the reactant side of a reaction, you must have ___ , ___ , and ____ on the product side. • The ONLY thing we can change ...
Organic Chemistry I: Contents
... Cyclic compounds and polygon formulas A compound such as CH3CH2CH2CH3 is said to have its carbon atoms connected in a chain. Carbon atoms can be joined together in rings as well as in chains; a compound with one or more rings is called a cyclic compound which is always represented by polygon ...
... Cyclic compounds and polygon formulas A compound such as CH3CH2CH2CH3 is said to have its carbon atoms connected in a chain. Carbon atoms can be joined together in rings as well as in chains; a compound with one or more rings is called a cyclic compound which is always represented by polygon ...
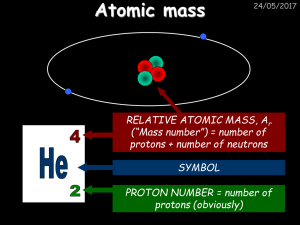
Atomic mass - drseemaljelani
... reaction, it is not always possible to obtain the calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the ...
... reaction, it is not always possible to obtain the calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the ...
Targets of Opportunity
... Yesterday, July 9, an explosion occurred at a Texas oil refinery which resulted in the release of an unspecified, but potentially large amount of hydrogen fluoride. According to the article which appeared in today's Corpus Christi Caller Times, a seven block area adjacent to the plant was soon evacu ...
... Yesterday, July 9, an explosion occurred at a Texas oil refinery which resulted in the release of an unspecified, but potentially large amount of hydrogen fluoride. According to the article which appeared in today's Corpus Christi Caller Times, a seven block area adjacent to the plant was soon evacu ...
Chem 1 Worksheets WSHEET 1: Working with Numbers Practice
... athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endothermic D. H > 0, process is endothermic E. H = 0, since cold packs are sealed 6. A Snickers® candy ...
... athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endothermic D. H > 0, process is endothermic E. H = 0, since cold packs are sealed 6. A Snickers® candy ...
C:\usb key\sch3u\unit 1\chapter 2 test answers.wpd
... In a co-ordinate bond, both electrons come from the same atom. 2) Draw a Lewis diagram for the molecule HO. Label one of each of the following types of electrons: lone pair, bonded pair, and unpaired (3 points). ...
... In a co-ordinate bond, both electrons come from the same atom. 2) Draw a Lewis diagram for the molecule HO. Label one of each of the following types of electrons: lone pair, bonded pair, and unpaired (3 points). ...
CHEM 150
... ____ 23. Which of the following molecules can have only London dispersion forces? a. CH4 b. CO2 c. both (a) and (b) d. neither (a) nor (b) ____ 24. Which of the following molecules cannot engage in hydrogen bonding? a. CH4 b. NH3 c. H2O d. all of them ____ 25. When comparing a liquid with a gas at t ...
... ____ 23. Which of the following molecules can have only London dispersion forces? a. CH4 b. CO2 c. both (a) and (b) d. neither (a) nor (b) ____ 24. Which of the following molecules cannot engage in hydrogen bonding? a. CH4 b. NH3 c. H2O d. all of them ____ 25. When comparing a liquid with a gas at t ...
(iii) Formation of Hydrogen chloride molecule
... oxygen to form magnesium oxide the two electrons in the outer shell of each magnesium atom are transferred to the incompletely filled orbitals of an oxygen atom. ...
... oxygen to form magnesium oxide the two electrons in the outer shell of each magnesium atom are transferred to the incompletely filled orbitals of an oxygen atom. ...
Chemical Bonds Study Guide Answer Key
... Define the following: 1. Chemical formula - the way of expressing information about the proportions of atoms that constitute a particular chemical compound, using element symbols and numbers. 2. Molecule- electrically neutral group of two or more atoms held together by chemical bonds 3. Valence elec ...
... Define the following: 1. Chemical formula - the way of expressing information about the proportions of atoms that constitute a particular chemical compound, using element symbols and numbers. 2. Molecule- electrically neutral group of two or more atoms held together by chemical bonds 3. Valence elec ...
Unit 4: Chemical Bonding Notes Chemical Bond—a mutual
... that binds the atoms together. Chemical bonds create more stable arrangements of matter. The goal of any atom is to gain, lose, or share valence electrons creating chemical bonds to provide a mor ...
... that binds the atoms together. Chemical bonds create more stable arrangements of matter. The goal of any atom is to gain, lose, or share valence electrons creating chemical bonds to provide a mor ...
Similarities in the electrical properties of transition metal–hydrogen
... concentration depth profiles with Eq. (1) can be formed for the various complexes and provide an estimate of the number of H atoms. This means for platinum (Fig. 3) that E(250) has the largest penetration depth and is therefore assigned to a complex with only one hydrogen atom. E(90) and H(210) exhi ...
... concentration depth profiles with Eq. (1) can be formed for the various complexes and provide an estimate of the number of H atoms. This means for platinum (Fig. 3) that E(250) has the largest penetration depth and is therefore assigned to a complex with only one hydrogen atom. E(90) and H(210) exhi ...
Kinds and Characteristics of Hydrogen Storage Alloy
... The first group is the combination of alkali earth metal A and transition metal B in the form of A2B, as represented by Mg2Ni. All of alloys classified in this group have not always identical crystal structures, and hydrides formed may have different structures. However, all of alloys contain magnes ...
... The first group is the combination of alkali earth metal A and transition metal B in the form of A2B, as represented by Mg2Ni. All of alloys classified in this group have not always identical crystal structures, and hydrides formed may have different structures. However, all of alloys contain magnes ...
Common Chemical Formula List
... has remained intact, then that can often be balanced first, as it is acts as a single species. The ions NO3- and CO32- are examples of a complex ion. A VERY useful rule is to leave balancing oxygen and hydrogen to the last steps as these elements are often in more than one chemical on each side , an ...
... has remained intact, then that can often be balanced first, as it is acts as a single species. The ions NO3- and CO32- are examples of a complex ion. A VERY useful rule is to leave balancing oxygen and hydrogen to the last steps as these elements are often in more than one chemical on each side , an ...
File
... electrons through chemical reactions. – This gives them an outer shell configuration like their nearest noble gas and therefore they become stable. – From the family number of the representative elements, you can determine the number of valence electrons, and therefore the number of electrons necess ...
... electrons through chemical reactions. – This gives them an outer shell configuration like their nearest noble gas and therefore they become stable. – From the family number of the representative elements, you can determine the number of valence electrons, and therefore the number of electrons necess ...
2015 AP Chemistry Summer Assignment
... a) The atom can be broken down into smaller parts. What are the smaller parts? b) How are atoms of hydrogen identical to each other, and how can they be different from each other? c) How are atoms of hydrogen different from atoms of helium? How can H atoms be similar to He atoms? d) How is water dif ...
... a) The atom can be broken down into smaller parts. What are the smaller parts? b) How are atoms of hydrogen identical to each other, and how can they be different from each other? c) How are atoms of hydrogen different from atoms of helium? How can H atoms be similar to He atoms? d) How is water dif ...
Bonding. A. Ionic bonds form when anions and cations arise
... Ionic bonds form when anions and cations arise from a transfer of electrons. 1. The transfer of electrons can occur if the two atoms have complementary octet rules. Consider the case of common table salt, sodium chloride. a) The octet rule for sodium is to lose one electron. b) The octet rule for ch ...
... Ionic bonds form when anions and cations arise from a transfer of electrons. 1. The transfer of electrons can occur if the two atoms have complementary octet rules. Consider the case of common table salt, sodium chloride. a) The octet rule for sodium is to lose one electron. b) The octet rule for ch ...
Hydrogen bond
A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (H) atom bound to a highly electronegative atom such as nitrogen (N), oxygen (O) or fluorine (F) experiences attraction to some other nearby highly electronegative atom.These hydrogen-bond attractions can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular). The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have no hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.In 2011, an IUPAC Task Group recommended a modern evidence-based definition of hydrogen bonding, which was published in the IUPAC journal Pure and Applied Chemistry. This definition specifies that The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. An accompanying detailed technical report provides the rationale behind the new definition.