Ionic bonding
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
C2 Revision Quick Questions FT
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
C2 Revision Quick Questions FT
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
C2 revision slides V3 + questions + MS – F
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
... Catalyst: Catalysts change the rate of chemical reactions but are not used up during the reaction. Different reactions need different catalysts. Catalysts are important in increasing the rates of chemical reactions used in industrial processes to reduce costs. Pressure: Increasing pressure increases ...
Lecture 4
... Important exceptions are acids and compounds such as ammonia that react with water to form ions. Strong and Weak Electrolytes Strong electrolytes exist in solution completely or nearly completely as ions. Weak electrolytes produce small concentrations of ions when they dissolve. Do not confuse the e ...
... Important exceptions are acids and compounds such as ammonia that react with water to form ions. Strong and Weak Electrolytes Strong electrolytes exist in solution completely or nearly completely as ions. Weak electrolytes produce small concentrations of ions when they dissolve. Do not confuse the e ...
makeup6
... 12. Which of the following statements about ionic compounds is false? (A) Ionic compounds are hard, brittle solids with high melting points. (B) Some compounds which exhibit primarily ionic bonding in the solid phase can form covalent bonds in the gas phase. (C) The lattice energy is the quantity of ...
... 12. Which of the following statements about ionic compounds is false? (A) Ionic compounds are hard, brittle solids with high melting points. (B) Some compounds which exhibit primarily ionic bonding in the solid phase can form covalent bonds in the gas phase. (C) The lattice energy is the quantity of ...
PowerPoint Lectures - Northwest ISD Moodle
... In an acid-base (neutralization) reaction, the acid donates a proton (H+) to the base. Generally, when solutions of an acid and a base are combined, the products are a salt (ionic compound) and water. - Water is neutral on the pH scale, unlike acids or bases, hence the name neutralization reaction - ...
... In an acid-base (neutralization) reaction, the acid donates a proton (H+) to the base. Generally, when solutions of an acid and a base are combined, the products are a salt (ionic compound) and water. - Water is neutral on the pH scale, unlike acids or bases, hence the name neutralization reaction - ...
Question paper - Edexcel
... SECTION A Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with If you change your mind, put a line through the box a cross . 1 The r ...
... SECTION A Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with If you change your mind, put a line through the box a cross . 1 The r ...
Lecture 25 Notes
... Brønsted-Lowry acids and bases A Brønsted-Lowry acid is any substance that is able to give hydrogen ions (H+) to another molecule or ion ...
... Brønsted-Lowry acids and bases A Brønsted-Lowry acid is any substance that is able to give hydrogen ions (H+) to another molecule or ion ...
Equation Writing Information
... reacts with a base (salt and water being formed). Example Write the net ionic equation for the reaction of sulphur dioxide gas with sodium hydroxide solution Answer: SO2(g) + 2OH-(aq) ---> SO32-(aq) + H 2O MISCELLANEOUS REACTIONS INVOLVING NaOH AND KOH KOH(aq) and Hydrogen Sulphide (H2S) gas Hydroge ...
... reacts with a base (salt and water being formed). Example Write the net ionic equation for the reaction of sulphur dioxide gas with sodium hydroxide solution Answer: SO2(g) + 2OH-(aq) ---> SO32-(aq) + H 2O MISCELLANEOUS REACTIONS INVOLVING NaOH AND KOH KOH(aq) and Hydrogen Sulphide (H2S) gas Hydroge ...
File
... The reactivity of alkali metals decreases going down the group. What is the reason for this? The atoms of each element get F larger going down the group. This means that the outer shell gets further away from the nucleus and is shielded by more electron shells. Cl The further the outer shell ...
... The reactivity of alkali metals decreases going down the group. What is the reason for this? The atoms of each element get F larger going down the group. This means that the outer shell gets further away from the nucleus and is shielded by more electron shells. Cl The further the outer shell ...
Chemistry Review
... Determine the charge of each (you should know most of them!) CRISS-CROSS the charges. IF (and only if) you add a subscript after a polyatomic ion, you need to put the polyatomic ion in parentheses. ...
... Determine the charge of each (you should know most of them!) CRISS-CROSS the charges. IF (and only if) you add a subscript after a polyatomic ion, you need to put the polyatomic ion in parentheses. ...
Unit F335/01
... (a) Suggest the formula of one functional group on the dye that makes it more soluble in water. Explain why your suggested group does this. ...
... (a) Suggest the formula of one functional group on the dye that makes it more soluble in water. Explain why your suggested group does this. ...
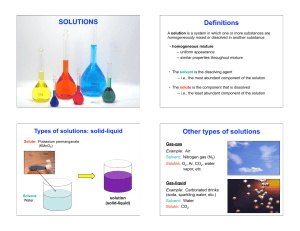
Solution
... What are the characteristics of a Brønsted acid? Does it contain at least an H atom? With the exception of ammonia, most Brønsted bases that you will encounter at this stage are anions. ...
... What are the characteristics of a Brønsted acid? Does it contain at least an H atom? With the exception of ammonia, most Brønsted bases that you will encounter at this stage are anions. ...
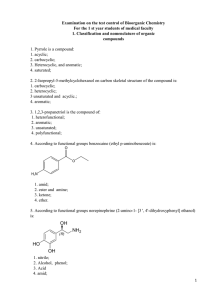
2. 2-Isopropyl-5-methylcyclohexanol on carbon skeletal
... 1. OH- group consisting of carboxylic acid functional groups; 2. hydroxyl group with sp 3-hybrid oxygen; 3. N-H acidic center; 4. OH group bonded directly to the heterocycle; 39. The most strong acidic properties of the compound shown in: 1. acetic acid; 2. propanoic acid; 3. 2-methylpropanoic acid; ...
... 1. OH- group consisting of carboxylic acid functional groups; 2. hydroxyl group with sp 3-hybrid oxygen; 3. N-H acidic center; 4. OH group bonded directly to the heterocycle; 39. The most strong acidic properties of the compound shown in: 1. acetic acid; 2. propanoic acid; 3. 2-methylpropanoic acid; ...
Name ______ Write formulas for the reactants and predicted
... hydrated ions acceptable with correct charge; partial credit for Ni(OH)2 as product ...
... hydrated ions acceptable with correct charge; partial credit for Ni(OH)2 as product ...
Unit 3 Revision Notes 213.00KB 2017-03-01 18
... NH3 + H2O = NH4+(aq) + OH-(aq) (the ionisation reaction is reversible) When an acid reacts with an alkali, the ionic equation is: H+ + OH- = H2O This is the ionic equation for all acid - alkali reactions. Choice of Indicators for Titrations The volumes of solution which react with each other can be ...
... NH3 + H2O = NH4+(aq) + OH-(aq) (the ionisation reaction is reversible) When an acid reacts with an alkali, the ionic equation is: H+ + OH- = H2O This is the ionic equation for all acid - alkali reactions. Choice of Indicators for Titrations The volumes of solution which react with each other can be ...
Net ionic equation
... • Water is a poor conductor of electricity • Aqueous solutions of ions can conduct electricity. • Three types of solutes: • Strong electrolytes: (solute is all ions) • Weak electrolytes: (some ions, mostly molecules) ...
... • Water is a poor conductor of electricity • Aqueous solutions of ions can conduct electricity. • Three types of solutes: • Strong electrolytes: (solute is all ions) • Weak electrolytes: (some ions, mostly molecules) ...
Chapter1 - WilsonChemWiki
... Ions: are atoms or group of atoms that have lost or gained electrons, and are classified according to their charges to: Positive ions (Cations): are atoms that have lost electron(s). Like; Li loses 1e- and become Li+ ion, Ca loses 2e- and become Ca+2 ion, Al loses 3e- and become Al+3 ion,… Negative ...
... Ions: are atoms or group of atoms that have lost or gained electrons, and are classified according to their charges to: Positive ions (Cations): are atoms that have lost electron(s). Like; Li loses 1e- and become Li+ ion, Ca loses 2e- and become Ca+2 ion, Al loses 3e- and become Al+3 ion,… Negative ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.