CHEM*130 (F 01) REVIEW QUESTIONS FOR MIDTERM I PAGE
... When aluminum metal is dissolved in perchloric acid, aluminum (III) perchlorate and hydrogen gas are formed. In the balanced equation for this reaction, what are the coefficients of hydrogen gas and perchloric acid, respectively? ...
... When aluminum metal is dissolved in perchloric acid, aluminum (III) perchlorate and hydrogen gas are formed. In the balanced equation for this reaction, what are the coefficients of hydrogen gas and perchloric acid, respectively? ...
Topic 4 - Lloyd Crosby
... b. Most acids are weak electrolytes Important exceptions are strong acids: HCl HBr HI HNO3 H2SO4 HClO4 c. Group I A hydroxides and Group II A hydroxides (from Ca on) are strong electrolytes. d. Most other substances are nonelectrolytes. B. Nonelectrolytes 1. Definition A substance that does not ioni ...
... b. Most acids are weak electrolytes Important exceptions are strong acids: HCl HBr HI HNO3 H2SO4 HClO4 c. Group I A hydroxides and Group II A hydroxides (from Ca on) are strong electrolytes. d. Most other substances are nonelectrolytes. B. Nonelectrolytes 1. Definition A substance that does not ioni ...
oxidation and reduction
... c) For each of the following, state whether or not it is a redox reaction (Y/N) and give a reason if it is or a reaction type if not: ...
... c) For each of the following, state whether or not it is a redox reaction (Y/N) and give a reason if it is or a reaction type if not: ...
AQA Additional Sci C2 Revision Guide
... form positively charged ions. Non-metal atoms gain electrons to form negatively charged ions. Ions have the electronic structure of a noble gas i.e. they have full outer shells. Oppositely charged ions are strongly attracted to each other and are held together by ionic bonds. The diagram below shows ...
... form positively charged ions. Non-metal atoms gain electrons to form negatively charged ions. Ions have the electronic structure of a noble gas i.e. they have full outer shells. Oppositely charged ions are strongly attracted to each other and are held together by ionic bonds. The diagram below shows ...
Chemistry Answers - Heathcote School and Science College
... a Calculate the maximum theoretical mass of hydrazine that can be made by reacting 340 g of ammonia with an excess of sodium chlorate. ...
... a Calculate the maximum theoretical mass of hydrazine that can be made by reacting 340 g of ammonia with an excess of sodium chlorate. ...
Exam 1
... questions; unsimplified answers will not be given full marks. • show all working in your answers to numerical questions. No credit will be given for an incorrect answer unless it is accompanied by details of the working. • make sure chemical equations are balanced and that the formulas for individua ...
... questions; unsimplified answers will not be given full marks. • show all working in your answers to numerical questions. No credit will be given for an incorrect answer unless it is accompanied by details of the working. • make sure chemical equations are balanced and that the formulas for individua ...
Lesson 1 Reversible reactions and equilibrium
... 2. More soluble fertiliser – bad It will dissolve in rain and wash into local drains. 3. Avoid applying it before rain is due – good This means it won’t dissolve in the rain and wash into drains. 4. Grow a quick crop of legumes – good But depends on timings of crops, etc. 5. Use fertiliser with larg ...
... 2. More soluble fertiliser – bad It will dissolve in rain and wash into local drains. 3. Avoid applying it before rain is due – good This means it won’t dissolve in the rain and wash into drains. 4. Grow a quick crop of legumes – good But depends on timings of crops, etc. 5. Use fertiliser with larg ...
C:\My Documents\My Documents\Teaching\chem130\hunt
... When aluminum metal is dissolved in perchloric acid, aluminum (III) perchlorate and hydrogen gas are formed. In the balanced equation for this reaction, what are the coefficients of hydrogen gas and perchloric acid, respectively? ...
... When aluminum metal is dissolved in perchloric acid, aluminum (III) perchlorate and hydrogen gas are formed. In the balanced equation for this reaction, what are the coefficients of hydrogen gas and perchloric acid, respectively? ...
Topic 4 Chemistry of the Elements of the Main Group
... H can form hydrides (negatively charged H) with a whole range of polarity, from ionic hydrides Cs-H ( = 1.4) to non-polar covalent hydrides B-H ( = 0.1). H can form hydrogen compounds (H is positively charged) with a whole range of polarity, from ionic hydrogen halides HF ( = 1.9) to non-polar ...
... H can form hydrides (negatively charged H) with a whole range of polarity, from ionic hydrides Cs-H ( = 1.4) to non-polar covalent hydrides B-H ( = 0.1). H can form hydrogen compounds (H is positively charged) with a whole range of polarity, from ionic hydrogen halides HF ( = 1.9) to non-polar ...
handout 4
... Lecture Examples: In each of the following examples, determine has been oxidized and what has been reduced and which is the oxidizing agent and which is the reducing agent. a. Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g) ...
... Lecture Examples: In each of the following examples, determine has been oxidized and what has been reduced and which is the oxidizing agent and which is the reducing agent. a. Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g) ...
spring semester review
... 10. Cut volume by ½ 11. Increase Temperature 12. Add a catalyst 13. For which one of the following reactions is Kc equal to Kp? (a) H2(g) + Cl2(g) <--> 2HCl(g) (c) N2O4(g) <--> 2NO2(g) (b) 2SO3(g) <--> 2SO2(g) + O2(g) (d) C(s) + CO2(g) <--> 2CO(g) 14. Which of the following does NOT represent an aci ...
... 10. Cut volume by ½ 11. Increase Temperature 12. Add a catalyst 13. For which one of the following reactions is Kc equal to Kp? (a) H2(g) + Cl2(g) <--> 2HCl(g) (c) N2O4(g) <--> 2NO2(g) (b) 2SO3(g) <--> 2SO2(g) + O2(g) (d) C(s) + CO2(g) <--> 2CO(g) 14. Which of the following does NOT represent an aci ...
2nd Nine Weeks Notes
... 5. Knowing the rate law for a reaction is important mainly because we can usually infer the individual steps involved in the reaction from the specific form of the rate law. IV. Determining the Form of the Rate Law. A. Introduction. 1. The first step in understanding how a given chemical reaction oc ...
... 5. Knowing the rate law for a reaction is important mainly because we can usually infer the individual steps involved in the reaction from the specific form of the rate law. IV. Determining the Form of the Rate Law. A. Introduction. 1. The first step in understanding how a given chemical reaction oc ...
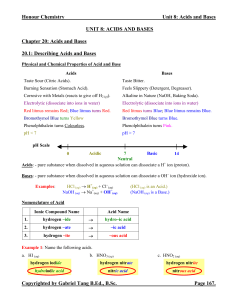
Unit 8 Acids and Bases Notes (answers)
... Polyprotic Acids: - acids that can donate more than one protons. - this includes all diprotic and triprotic acids (acids that can donate three protons). - polyprotic acids dissociate one proton at a time. Each successive proton donation has its own Ka, which gets smaller until the last proton is don ...
... Polyprotic Acids: - acids that can donate more than one protons. - this includes all diprotic and triprotic acids (acids that can donate three protons). - polyprotic acids dissociate one proton at a time. Each successive proton donation has its own Ka, which gets smaller until the last proton is don ...
Dr David`s Chemistry Test Answers
... 7. van der Waal - these are weak temporary dipole-dipole or dispersion forces. They are the result of electron oscillations in the large iodine molecules. 8. The lone pairs on the oxygen occupy their own This results in an angled space and repel one another & the bonding pairs. molecule rather than ...
... 7. van der Waal - these are weak temporary dipole-dipole or dispersion forces. They are the result of electron oscillations in the large iodine molecules. 8. The lone pairs on the oxygen occupy their own This results in an angled space and repel one another & the bonding pairs. molecule rather than ...
Qualitative Analysis of Anions
... Test for Sulfite Ion, SO3 Continuing with the sample used the sulfate test above….. Centrifuge the tube to obtain a clear filtrate. Add a drop or two of 0.1 M BaCl2 to be sure all of the 2SO4 has precipitated. Centrifuge again if more BaSO4 formed in the solution. Decant the filtrate into a new test ...
... Test for Sulfite Ion, SO3 Continuing with the sample used the sulfate test above….. Centrifuge the tube to obtain a clear filtrate. Add a drop or two of 0.1 M BaCl2 to be sure all of the 2SO4 has precipitated. Centrifuge again if more BaSO4 formed in the solution. Decant the filtrate into a new test ...
Chapter 1 questions
... e) Ca(OH)2 (aq) + CO2 (g) ----> Ca(HCO3)2 (aq) Q5. Write equations to show the successive ionisations of the triprotic acid H3PO4 reacting with water. Q6. State whether each of the following species undergoes ionisation or dissociation when added to water: a) Mg(OH)2 (s) b) KI (s) c) HCl (g) d) H2O ...
... e) Ca(OH)2 (aq) + CO2 (g) ----> Ca(HCO3)2 (aq) Q5. Write equations to show the successive ionisations of the triprotic acid H3PO4 reacting with water. Q6. State whether each of the following species undergoes ionisation or dissociation when added to water: a) Mg(OH)2 (s) b) KI (s) c) HCl (g) d) H2O ...
Unit-2-Hydrocarbons
... Alcohols and Carboxylic acids also have a hydroxyl group with a hydrogen bonded to an oxygen. This allows them to form hydrogen bonds with each other. Therefore, carboxylic acids have at least three different noncovalent interactions: ...
... Alcohols and Carboxylic acids also have a hydroxyl group with a hydrogen bonded to an oxygen. This allows them to form hydrogen bonds with each other. Therefore, carboxylic acids have at least three different noncovalent interactions: ...
Exam - Vcaa
... will determine if a back titration is to be used. Consider the following cases. I The substance being analysed is volatile. II The substance being analysed is insoluble in water but is soluble in dilute acid. III The end point of the reaction is difficult to detect. In which cases would a back titra ...
... will determine if a back titration is to be used. Consider the following cases. I The substance being analysed is volatile. II The substance being analysed is insoluble in water but is soluble in dilute acid. III The end point of the reaction is difficult to detect. In which cases would a back titra ...
Otto F. Meyerhof - Nobel Lecture
... of glycogen is assumed, of which four are esterified with phosphoric acid and form eight molecules of lactic acid. In the second, aerobic phase these eight molecules of lactic acid disappear, while two of them, or alternatively, as we might equally well assume, one molecule of sugar, are burnt. The ...
... of glycogen is assumed, of which four are esterified with phosphoric acid and form eight molecules of lactic acid. In the second, aerobic phase these eight molecules of lactic acid disappear, while two of them, or alternatively, as we might equally well assume, one molecule of sugar, are burnt. The ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.