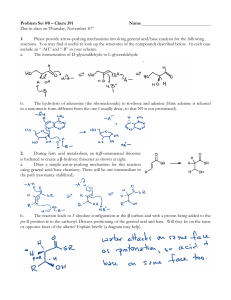
Acidic and Basic Character of Carboxylic Acids
... Electron-withdrawing substituents increase the acidity of carboxylic acids. The inductive effect of electron-withdrawing groups close to the carboxy group causes an increase in acidity. Three electron-withdrawing groups on the -carbon sometimes results in acidity near that of some inorganic acids. ...
... Electron-withdrawing substituents increase the acidity of carboxylic acids. The inductive effect of electron-withdrawing groups close to the carboxy group causes an increase in acidity. Three electron-withdrawing groups on the -carbon sometimes results in acidity near that of some inorganic acids. ...
Chapter 1
... – Carbon: normally forms four covalent bonds and has no unshared pairs of electrons. C – Hydrogen: forms one covalent bond and no unshared pairs of electrons. H – Nitrogen: normally forms three covalent bonds and has one unshared pair of electrons. ...
... – Carbon: normally forms four covalent bonds and has no unshared pairs of electrons. C – Hydrogen: forms one covalent bond and no unshared pairs of electrons. H – Nitrogen: normally forms three covalent bonds and has one unshared pair of electrons. ...
1 - TEST BANK 360
... D) The forward and reverse reactions are equally favored. 49. Which species is the strongest base? A) NH3 C) OH– B) NH2– D) H– Challenge Questions 50. Amino acids exist in different charged forms depending on the pH of the solution. Leucine has two acidic protons with pKa values of 2.33 and 9.74 and ...
... D) The forward and reverse reactions are equally favored. 49. Which species is the strongest base? A) NH3 C) OH– B) NH2– D) H– Challenge Questions 50. Amino acids exist in different charged forms depending on the pH of the solution. Leucine has two acidic protons with pKa values of 2.33 and 9.74 and ...
JUNIOR COLLEGE CHEMISTRY DEPARTMENT EXPERIMENT 15
... Comment: The deactivating COOH would have given a buff precipitate with FeCl3 while the phenol gives a strongly coloured solution. FF, with both types of groups, gives the formation of a brightly coloured solution. The activating group chemistry i.e. that of the OH dominates over that of the deactiv ...
... Comment: The deactivating COOH would have given a buff precipitate with FeCl3 while the phenol gives a strongly coloured solution. FF, with both types of groups, gives the formation of a brightly coloured solution. The activating group chemistry i.e. that of the OH dominates over that of the deactiv ...
Chpt. 22: Some Families of Organic Compounds
... to a water molecule with an alkyl group replacing a hydrogen atom • The higher boiling points are due to the fact that the highly polar –OH group gives rise to hydrogen bonding between the alcohol molecules. Oxygen, being more electronegative, has a partial negative charge, and hydrogen has a partia ...
... to a water molecule with an alkyl group replacing a hydrogen atom • The higher boiling points are due to the fact that the highly polar –OH group gives rise to hydrogen bonding between the alcohol molecules. Oxygen, being more electronegative, has a partial negative charge, and hydrogen has a partia ...
Organic Chemistry
... Organic Chemistry The study of carbon containing compounds. All atoms are bonded covalently Why is Carbon so special? ...
... Organic Chemistry The study of carbon containing compounds. All atoms are bonded covalently Why is Carbon so special? ...
CARBONYL COMPOUNDS ALDEHYDES AND KETONES
... 1. Oxidation of Alcohols Primary alcohols can be oxidized with pyridinium chlorochromate (PCC) to aldehydes. Ketones can be obtained from secondary alcohols by oxidation with sodium dichromate/sulfuric acid or KMnO4. ...
... 1. Oxidation of Alcohols Primary alcohols can be oxidized with pyridinium chlorochromate (PCC) to aldehydes. Ketones can be obtained from secondary alcohols by oxidation with sodium dichromate/sulfuric acid or KMnO4. ...
22-2 Alcohols, Ethers, and Amines
... • Denatured alcohol has aviation gas or other solvents added to it to make it unfit to drink. • Ethanol is found in alcoholic beverages. • Ethanol and CO2 are produced by yeast when they ferment sugars, such as those in grapes and bread dough. • To name alcohols, use a number or numbers, if needed t ...
... • Denatured alcohol has aviation gas or other solvents added to it to make it unfit to drink. • Ethanol is found in alcoholic beverages. • Ethanol and CO2 are produced by yeast when they ferment sugars, such as those in grapes and bread dough. • To name alcohols, use a number or numbers, if needed t ...
classification of matter
... At the time, scientists believed that a “vital force”, only present in living organisms, was necessary to produce organic compounds. ...
... At the time, scientists believed that a “vital force”, only present in living organisms, was necessary to produce organic compounds. ...
친환경 촉매 Iron (III) phosphate: 실온/무용매 반응조건에서 알코올과
... Interestingly, phenols too underwent acetylation smoothly under the same reaction condition. Phenols with activating and deactivating groups altogether carried out acetylation rapidly with very high yield (entries 19-25). Strongly electron-withdrawing group such as nitro substituent produces 95% yie ...
... Interestingly, phenols too underwent acetylation smoothly under the same reaction condition. Phenols with activating and deactivating groups altogether carried out acetylation rapidly with very high yield (entries 19-25). Strongly electron-withdrawing group such as nitro substituent produces 95% yie ...
Lab 6
... directly bonded to an arene ring such as benzene. An alcohol contains a hydroxyl group bonded to a non-aromatic carbon atom, normally an sp3hybridized carbon atom. ...
... directly bonded to an arene ring such as benzene. An alcohol contains a hydroxyl group bonded to a non-aromatic carbon atom, normally an sp3hybridized carbon atom. ...
Solutions
... ∆H≠: Arguably Tyr is a superior general acid to water or acetic acid because phenols are better matched in pKa to the protonated intermediate. ∆S≠: Pre-organized relationship of GA & GB in active site removes entropic loss suffered by acetate in solution. ...
... ∆H≠: Arguably Tyr is a superior general acid to water or acetic acid because phenols are better matched in pKa to the protonated intermediate. ∆S≠: Pre-organized relationship of GA & GB in active site removes entropic loss suffered by acetate in solution. ...
Learning Check
... in the presence of water and heat. What will be the products of this reaction? To write the hydrolysis products, separate the compound at the ester bond. Complete the formula of the carboxylic acid by adding –OH (from water) to the carbonyl group and –H (from water) to the alcohol. ...
... in the presence of water and heat. What will be the products of this reaction? To write the hydrolysis products, separate the compound at the ester bond. Complete the formula of the carboxylic acid by adding –OH (from water) to the carbonyl group and –H (from water) to the alcohol. ...
Alcohols
... Phenols and thiols are more acidic than alcohols • Both are soluble in dilute aqueous NaOH • Can often be separated from a mixture by basic extraction into aqueous solution, followed by reacidification Phenols are more acidic than alcohols because the phenoxide anion is resonance-stabilized • Deloca ...
... Phenols and thiols are more acidic than alcohols • Both are soluble in dilute aqueous NaOH • Can often be separated from a mixture by basic extraction into aqueous solution, followed by reacidification Phenols are more acidic than alcohols because the phenoxide anion is resonance-stabilized • Deloca ...
Lecture_Syllabus_Gillies - Kingsborough Community College
... 15. That esters like Nitroglycerin which are the product of an organic alcohol and an acid, have beneficial use such as relieving angina. Phenyl mercuric acetate is used to disinfect instruments and as an antiseptic on cutaneous and mucosal surfaces. ...
... 15. That esters like Nitroglycerin which are the product of an organic alcohol and an acid, have beneficial use such as relieving angina. Phenyl mercuric acetate is used to disinfect instruments and as an antiseptic on cutaneous and mucosal surfaces. ...
2130404 - Gujarat Technological University
... Minimum 5 practicals to be performed and remaining Open-ended Projects / Study Reports / Latest outcomes in technology study :1. In the beginning of the academic term, faculties will have to allot their students at least one Projects / Study Reports / Latest outcomes in technology. 2. Literature sur ...
... Minimum 5 practicals to be performed and remaining Open-ended Projects / Study Reports / Latest outcomes in technology study :1. In the beginning of the academic term, faculties will have to allot their students at least one Projects / Study Reports / Latest outcomes in technology. 2. Literature sur ...
04_Lecture_Presentation
... • Isomers are compounds with the same molecular formula but different structures and properties – Structural isomers have different covalent arrangements of their atoms – Cis-trans isomers have the same covalent bonds but differ in spatial arrangements – Enantiomers are isomers that are mirror image ...
... • Isomers are compounds with the same molecular formula but different structures and properties – Structural isomers have different covalent arrangements of their atoms – Cis-trans isomers have the same covalent bonds but differ in spatial arrangements – Enantiomers are isomers that are mirror image ...
Chromatography Spectroscopy HW
... Explain why electrophiles, such as bromine, react much more readily with phenol than with benzene. ...
... Explain why electrophiles, such as bromine, react much more readily with phenol than with benzene. ...
Arenes HW
... Explain why electrophiles, such as bromine, react much more readily with phenol than with benzene. ...
... Explain why electrophiles, such as bromine, react much more readily with phenol than with benzene. ...
Organic Compounds Containing C, H and O
... Ans. i. a. Nitro (-NO2) group is an electron withdrawing whereas methoxy (-OCH3) group is electron releasing in nature. o-nitrophenol produces H+ ions easily but methoxyphenol does not. This is because o-nitrophenoxide ion is stabilised due to resonance. This is not true with o-methoxyphenoxide ion. ...
... Ans. i. a. Nitro (-NO2) group is an electron withdrawing whereas methoxy (-OCH3) group is electron releasing in nature. o-nitrophenol produces H+ ions easily but methoxyphenol does not. This is because o-nitrophenoxide ion is stabilised due to resonance. This is not true with o-methoxyphenoxide ion. ...
Organic Chemistry : Ch. 19
... Secondary, and tertiary. These types of alcohols are determined by “where” the OH is on the carbon chain. Primary : OH is at the end of a carbon chain. Secondary: OH is in the middle, with 2 C atoms attached to the C with the OH. Tertiary : OH is in the middle with 3 C atoms attached to the C ...
... Secondary, and tertiary. These types of alcohols are determined by “where” the OH is on the carbon chain. Primary : OH is at the end of a carbon chain. Secondary: OH is in the middle, with 2 C atoms attached to the C with the OH. Tertiary : OH is in the middle with 3 C atoms attached to the C ...
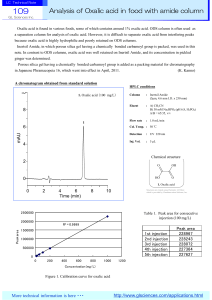
Analysis of Oxalic acid in food with amide column
... id column l is i usually ll usedd in i HILIC mode. d Salts S lt soluble l bl nott only l in i water t but b t also l in i organic i solvent l t are recommended for the mobile phase because organic solvent content is quite high in HILIC mode. <Recommended salts and their concentration> Ammonium aceta ...
... id column l is i usually ll usedd in i HILIC mode. d Salts S lt soluble l bl nott only l in i water t but b t also l in i organic i solvent l t are recommended for the mobile phase because organic solvent content is quite high in HILIC mode. <Recommended salts and their concentration> Ammonium aceta ...
Survival Organic Chemistry Molecular Models The goal in this
... out that you do not make a conformer, which is a different special arrangement of a compound but contains the same arrangement of carboncarbon bonds. Use your sheet of paper to draw the Lewis structure for each of the compounds and any structural isomers they may have. 3. Draw 4 structural isomers o ...
... out that you do not make a conformer, which is a different special arrangement of a compound but contains the same arrangement of carboncarbon bonds. Use your sheet of paper to draw the Lewis structure for each of the compounds and any structural isomers they may have. 3. Draw 4 structural isomers o ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.