Photoelectron spectroscopy of jet
... Pulsed UV laser light (hv=3.68 eV) is utilized to detach electrons from mass-identified cluster anion bunches. In Fig. 3 a, b we present the PE spectra of negatively charged A1, in the size range of 225 atoms. As usually, the data are plotted as a function of electron binding energy, given by the ph ...
... Pulsed UV laser light (hv=3.68 eV) is utilized to detach electrons from mass-identified cluster anion bunches. In Fig. 3 a, b we present the PE spectra of negatively charged A1, in the size range of 225 atoms. As usually, the data are plotted as a function of electron binding energy, given by the ph ...
Section 2.6
... • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions ...
... • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions ...
Types of bonds possible from Ligands
... Problem is their weak kinetic stability (Thermally fine: M-C bond dissociation energies are typically 4060 kcal/mol with 20-70 kcal/mol) Simple alkyls are sigma donors, that can be considered to donate one or two electrons to the metal center depending on which electron ...
... Problem is their weak kinetic stability (Thermally fine: M-C bond dissociation energies are typically 4060 kcal/mol with 20-70 kcal/mol) Simple alkyls are sigma donors, that can be considered to donate one or two electrons to the metal center depending on which electron ...
Notes on Atoms and Molecules
... Valency: The combining capacity of an element is known as valency. The combining capacity of the atoms to form molecules either with same or different elements is defined as valency. Atom contains less than four electrons in its outermost shell; the valency of an atom is equal to the number of elect ...
... Valency: The combining capacity of an element is known as valency. The combining capacity of the atoms to form molecules either with same or different elements is defined as valency. Atom contains less than four electrons in its outermost shell; the valency of an atom is equal to the number of elect ...
03 Complexation equilibrium
... (from left to right). The two solutions on the right were prepared by adding ammonia and ethylenediamine, respectively, to aqueous nickel(II) nitrate. ...
... (from left to right). The two solutions on the right were prepared by adding ammonia and ethylenediamine, respectively, to aqueous nickel(II) nitrate. ...
Whited Lit Discussion - M-L Multiple Bonds and FLPs
... interactions in the DCD model. Why is it important to have both filled and empty d orbitals cooperating in the DCD model? 2) Frustrated Lewis pairs (FLPs) have also been shown to activate molecules like H2 and alkenes without the use of transition metals. What are FLPs? Provide one example (from thi ...
... interactions in the DCD model. Why is it important to have both filled and empty d orbitals cooperating in the DCD model? 2) Frustrated Lewis pairs (FLPs) have also been shown to activate molecules like H2 and alkenes without the use of transition metals. What are FLPs? Provide one example (from thi ...
Coordination compounds
... d. The coordination number depends on the size, charge and electron configuration of the transition metal ion. e. Coordination number tells the shape of the complex ion. Similarly to VSEPR i. 6 = octahedral ii. 4= tetrahedral or square planar (no way to predict reliably) ...
... d. The coordination number depends on the size, charge and electron configuration of the transition metal ion. e. Coordination number tells the shape of the complex ion. Similarly to VSEPR i. 6 = octahedral ii. 4= tetrahedral or square planar (no way to predict reliably) ...
OMC-Karlin-Spring-`09-Lecture-Set
... stability.”, later accorded to the aromatic character of Cp– groups. The sandwich compound structure was described later; this led to new metallocenes chemistry (1973 Nobel prize, Wilkinson & Fischer). The Fe atom is assigned to the +2 oxidation state (Mössbauer spectroscopy). The bonding nature in ...
... stability.”, later accorded to the aromatic character of Cp– groups. The sandwich compound structure was described later; this led to new metallocenes chemistry (1973 Nobel prize, Wilkinson & Fischer). The Fe atom is assigned to the +2 oxidation state (Mössbauer spectroscopy). The bonding nature in ...
This is an overview of what can happen during the
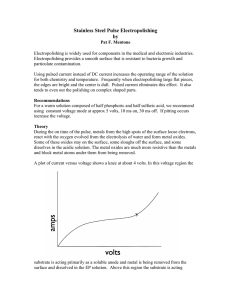
... primarily as an insoluble anode and the water is being electrolyzed to oxygen gas and hydrogen ions. In order for polishing to occur, the current and voltage must be above the knee of the curve, this location is marked with an X in the above graph If the substrate functioned as a 100% soluble anode ...
... primarily as an insoluble anode and the water is being electrolyzed to oxygen gas and hydrogen ions. In order for polishing to occur, the current and voltage must be above the knee of the curve, this location is marked with an X in the above graph If the substrate functioned as a 100% soluble anode ...
Complexes_naming(download)
... (a) The structure of the porphine molecule. Loss of the two NH protons gives a planar, tetradentate 2– ligand that can bond to a metal cation. The porphyrins are derivatives of porphine in which the peripheral H atoms are replaced by various substituent groups. (b) Schematic of the planar heme group ...
... (a) The structure of the porphine molecule. Loss of the two NH protons gives a planar, tetradentate 2– ligand that can bond to a metal cation. The porphyrins are derivatives of porphine in which the peripheral H atoms are replaced by various substituent groups. (b) Schematic of the planar heme group ...
Chemistry Scavenger Hunt
... 2. There are three main phases of matter: _____________, ____________, and _____________. There is also a fourth phase, ______________, but it exists at very high temperatures. Science Is Fun 2 Go to the “ChemTime Clock” area to find the answers to these questions. http://scifun.chem.wisc.edu/ChemTi ...
... 2. There are three main phases of matter: _____________, ____________, and _____________. There is also a fourth phase, ______________, but it exists at very high temperatures. Science Is Fun 2 Go to the “ChemTime Clock” area to find the answers to these questions. http://scifun.chem.wisc.edu/ChemTi ...
communications - University of California, Berkeley
... solution of all possible isomers (with point group symmetry): DDDD (T), DDDL (C3), DDLL (S4), and their mirror images. In contrast, the tetrahedral complex [Ga426] has strongly coupled metal centers, such that if one metal center initially has a L configuration, the metal center across an edge from ...
... solution of all possible isomers (with point group symmetry): DDDD (T), DDDL (C3), DDLL (S4), and their mirror images. In contrast, the tetrahedral complex [Ga426] has strongly coupled metal centers, such that if one metal center initially has a L configuration, the metal center across an edge from ...
Isomerism of coordination compounds
... Isomerism of coordination compounds Structural isomerism Ionization isomerism When ligands are exchanged in a complex by the counter-ion, it is called ionization isomerism. e.g. [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl Linkage isomerism A ligand connects with the central metal atom through different ato ...
... Isomerism of coordination compounds Structural isomerism Ionization isomerism When ligands are exchanged in a complex by the counter-ion, it is called ionization isomerism. e.g. [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl Linkage isomerism A ligand connects with the central metal atom through different ato ...
Atomic arrangement, short and long range order, point. Direction
... ree of ordering of an alloy— for example. in an alloy consisting of twocomponents, complete ordering leads to a lternation of the two types of atoms; in other words, thenearest neighbors of each at om are only atoms of the other type. Incomplete order is reflected bythe fact that at oms of the same ...
... ree of ordering of an alloy— for example. in an alloy consisting of twocomponents, complete ordering leads to a lternation of the two types of atoms; in other words, thenearest neighbors of each at om are only atoms of the other type. Incomplete order is reflected bythe fact that at oms of the same ...
Stereochemistry of hexacoordinated transition metal complexes with
... Hexacoordinated transition metal complexes of tridentate ligands L with ML2 stoichiometry can form several geometrical isomers: meridional, trans-facial, and delta or lambda cis-facial. The configuration of isomers is affected by steric and electronic properties of the ligands and coordinating abili ...
... Hexacoordinated transition metal complexes of tridentate ligands L with ML2 stoichiometry can form several geometrical isomers: meridional, trans-facial, and delta or lambda cis-facial. The configuration of isomers is affected by steric and electronic properties of the ligands and coordinating abili ...
Chapter 19, Coordination and Organometallic Compounds
... Organometallic Compounds Hapticities the Hapto Nomenclature ...
... Organometallic Compounds Hapticities the Hapto Nomenclature ...
Other useful things to know about atoms
... have only weak attractions between the molecules. They will be volatile solid and liquids or already be a gas at room temperature. o Some form giant molecules, especially silicon – the element of the rocks. o Carbon atoms are unique in that they form strong carbon-carbon bonds allowing the formation ...
... have only weak attractions between the molecules. They will be volatile solid and liquids or already be a gas at room temperature. o Some form giant molecules, especially silicon – the element of the rocks. o Carbon atoms are unique in that they form strong carbon-carbon bonds allowing the formation ...
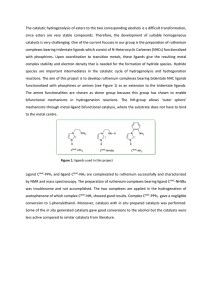
The catalytic hydrogenolysis of esters to the two corresponding
... The catalytic hydrogenolysis of esters to the two corresponding alcohols is a difficult transformation, since esters are very stable compounds. Therefore, the development of suitable homogeneous catalysts is very challenging. One of the current focuses in our group is the preparation of ruthenium co ...
... The catalytic hydrogenolysis of esters to the two corresponding alcohols is a difficult transformation, since esters are very stable compounds. Therefore, the development of suitable homogeneous catalysts is very challenging. One of the current focuses in our group is the preparation of ruthenium co ...
Chemical formula Chemistry Subscript Subscript
... 8U2 - 5(D) recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts; A way of describing the number of atoms Chemical formula that makes up one molecule of a compound ...
... 8U2 - 5(D) recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts; A way of describing the number of atoms Chemical formula that makes up one molecule of a compound ...
points defects - Fundamental Material Reviewers
... – Element or compound present in the greatest amount; also called host atoms ...
... – Element or compound present in the greatest amount; also called host atoms ...
Percent buried volume (%V bur )
... Poater, A.; Cosenza, B.; Correa, A.; Giudice, S.; Ragone, F.; Scarano, V.; Cavallo, L. Eur. J. Inorg. Chem. 2009, ...
... Poater, A.; Cosenza, B.; Correa, A.; Giudice, S.; Ragone, F.; Scarano, V.; Cavallo, L. Eur. J. Inorg. Chem. 2009, ...
role of the ancillary ligands on the coordination modes
... Nuovi farmaci inorganici in oncologia agreement with the formation of a single reaction product. In the 1H NMR spectrum, the presence of one of the NH resonances at δ 10.6 suggests that platination of the neutral nucleobase occurs at the N(4) atom, as result of a tautomeric shift of one of the exocy ...
... Nuovi farmaci inorganici in oncologia agreement with the formation of a single reaction product. In the 1H NMR spectrum, the presence of one of the NH resonances at δ 10.6 suggests that platination of the neutral nucleobase occurs at the N(4) atom, as result of a tautomeric shift of one of the exocy ...
2D-EE spectroscopy of heavier alkaline earth metals
... A two dimensional excitation-emission (2D-EE) spectroscopic investigation of Sr/RG and Ba/RG solids (RG = Ar, Kr and Xe) has revealed [1] the presence of these heavy alkaline earth atoms in unusual sites in the solid rare gases. Sr, identified as an impurity in the barium metal used, provided the in ...
... A two dimensional excitation-emission (2D-EE) spectroscopic investigation of Sr/RG and Ba/RG solids (RG = Ar, Kr and Xe) has revealed [1] the presence of these heavy alkaline earth atoms in unusual sites in the solid rare gases. Sr, identified as an impurity in the barium metal used, provided the in ...
Metal Carbonyls - TAMU Chemistry
... •Semibridging: somewhere between terminal and µ-2. Trends observed in the IR spectra of carbonyl complexes that are consistent with the concept of π-backbonding: 1.With each charge added to the metal center, the CO stretching frequency decreases by approximately 100 cm-1. 2. The better the sigma-don ...
... •Semibridging: somewhere between terminal and µ-2. Trends observed in the IR spectra of carbonyl complexes that are consistent with the concept of π-backbonding: 1.With each charge added to the metal center, the CO stretching frequency decreases by approximately 100 cm-1. 2. The better the sigma-don ...