Biochemistry of Biomolecules Page | 1 BIOCHEMISTRY OF
... The phospholipid bilayer is permeable to very small uncharged molecules like oxygen and carbon dioxide. Hydrophobic substances, for example, steroids can also diffuse through. The phospholipid bilayer is not permeable to charged ions and hydrophilic molecules like glucose and macromolecules. T ...
... The phospholipid bilayer is permeable to very small uncharged molecules like oxygen and carbon dioxide. Hydrophobic substances, for example, steroids can also diffuse through. The phospholipid bilayer is not permeable to charged ions and hydrophilic molecules like glucose and macromolecules. T ...
Isofocusing Chromatography
... within the pH gradient in order to avoid aggregation and precipitation of some proteins. This is only possible on a horizontal gel with an open surface. •Sharply focused bands can only be obtained with a high field strength, high voltages have to be applied. Only horizontal equipment can meet the ne ...
... within the pH gradient in order to avoid aggregation and precipitation of some proteins. This is only possible on a horizontal gel with an open surface. •Sharply focused bands can only be obtained with a high field strength, high voltages have to be applied. Only horizontal equipment can meet the ne ...
i principi di base - Structural Biology
... spectroscopy, observing the vibration band of the NH group, that is different depending on whether the molecule is in the monomeric or dimeric form (the proton, interacting with the carbonyl of another molecule, vibrates differently). ...
... spectroscopy, observing the vibration band of the NH group, that is different depending on whether the molecule is in the monomeric or dimeric form (the proton, interacting with the carbonyl of another molecule, vibrates differently). ...
Protein Story-telling S. Krishnaswamy, The Institute of Mathematical
... Protein Story-telling S. Krishnaswamy, The Institute of Mathematical Sciences, Chennai Proteins are molecules that are needed by humans and insects and all the other living things. Without these molecules, we will not live or function. They are the machines of the cells. . Proteins such as synthetas ...
... Protein Story-telling S. Krishnaswamy, The Institute of Mathematical Sciences, Chennai Proteins are molecules that are needed by humans and insects and all the other living things. Without these molecules, we will not live or function. They are the machines of the cells. . Proteins such as synthetas ...
Practice Exam-Final Fall 2016 W-Ans
... 16. How many hydrogen atoms are there in 48.0 g of CH4? (a) 1.81x1023 (b) 7.22x1024 (c) 6.02x1023 (d) 1.20x1025 (e) 4.70x1025 Hint: According to the chemical formula, one mole of CH4 contains 1 mole of C atoms and 4 moles of hydrogen atoms. Thus, the mole of H = 4 x {mass of CH4/molar mass of CH4}. ...
... 16. How many hydrogen atoms are there in 48.0 g of CH4? (a) 1.81x1023 (b) 7.22x1024 (c) 6.02x1023 (d) 1.20x1025 (e) 4.70x1025 Hint: According to the chemical formula, one mole of CH4 contains 1 mole of C atoms and 4 moles of hydrogen atoms. Thus, the mole of H = 4 x {mass of CH4/molar mass of CH4}. ...
Protein Domain Boundary Prediction
... • Domains provide one of the most valuable information for the prediction of protein structure, function, evolution and design. • Since Anfinsen’s (1973) seminal work, many have proposed various structure prediction models from amino acid sequence only. • This study, - Provides an overview of the mo ...
... • Domains provide one of the most valuable information for the prediction of protein structure, function, evolution and design. • Since Anfinsen’s (1973) seminal work, many have proposed various structure prediction models from amino acid sequence only. • This study, - Provides an overview of the mo ...
Introduction to Spectroscopy and Fluorescence
... Role in function: As it contains a non-carbon atom (nitrogen) in the aromatic ring system, Tryptophan is more reactive than Phenylalanine though it is less reactive than Tyrosine. The Tryptophan nitrogens can play a role in binding to non-protein atoms, but such instances are rare. ...
... Role in function: As it contains a non-carbon atom (nitrogen) in the aromatic ring system, Tryptophan is more reactive than Phenylalanine though it is less reactive than Tyrosine. The Tryptophan nitrogens can play a role in binding to non-protein atoms, but such instances are rare. ...
HS-LS1-1
... Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. [Clarification Statement: Emphasis is on using evidence from models and simulations to suppor ...
... Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. [Clarification Statement: Emphasis is on using evidence from models and simulations to suppor ...
Chapter 12 - Gordon State College
... • At 20 oC, the vapor pressure of pure benzene is 75 torr and that of pure toluene (C7H8) is 22 torr. Assume that benzene and toluene form an ideal solution. • A. What is the composition in mole fractions of a solution that has a vapor pressure of 35 torr at 20 oC? • B. What is the mole fraction of ...
... • At 20 oC, the vapor pressure of pure benzene is 75 torr and that of pure toluene (C7H8) is 22 torr. Assume that benzene and toluene form an ideal solution. • A. What is the composition in mole fractions of a solution that has a vapor pressure of 35 torr at 20 oC? • B. What is the mole fraction of ...
Name #_____
... 20. Given the following Lewis Dot structure, how many electrons are shared between AX? 21. Given the following Lewis Dot structures, how many lone pairs are around the central atom? A. ...
... 20. Given the following Lewis Dot structure, how many electrons are shared between AX? 21. Given the following Lewis Dot structures, how many lone pairs are around the central atom? A. ...
A typical hemoglobin molecule consists of a "heme" part and a
... A typical hemoglobin molecule consists of a "heme" part and a protein part=globin. The "heme" part lies in the interior of the hemoglobin molecule. At the center of the heme is an Fe(II), ferrous, atom. Four of the six coordination sites around this atom are occupied by nitrogen atoms from a porphyr ...
... A typical hemoglobin molecule consists of a "heme" part and a protein part=globin. The "heme" part lies in the interior of the hemoglobin molecule. At the center of the heme is an Fe(II), ferrous, atom. Four of the six coordination sites around this atom are occupied by nitrogen atoms from a porphyr ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... C. There are 8 geometric shapes of molecules: 1. Linear (Line in one plane) a. Number of atoms bonded to the central atom = 2. b. The molecule has a bond angle of 180O. c. It has a basic molecular formula of: AB2 (“A” is one element; “B” is the other element) For example: Be F2 2. Trigonal-Planer (3 ...
... C. There are 8 geometric shapes of molecules: 1. Linear (Line in one plane) a. Number of atoms bonded to the central atom = 2. b. The molecule has a bond angle of 180O. c. It has a basic molecular formula of: AB2 (“A” is one element; “B” is the other element) For example: Be F2 2. Trigonal-Planer (3 ...
FSTC 313
... Lipids are one of the major constituents of foods, and are important in our diet as a source of energy and essential lipid nutrients. In many foods the lipid component plays a major role in determining the overall physical characteristics, such as flavor, texture, mouthfeel and appearance. Lipids ar ...
... Lipids are one of the major constituents of foods, and are important in our diet as a source of energy and essential lipid nutrients. In many foods the lipid component plays a major role in determining the overall physical characteristics, such as flavor, texture, mouthfeel and appearance. Lipids ar ...
Final Exam Review Sheets
... c. Which substance is least affected by temperature change? ____ NaCl _________ d. Which substance is the least soluble in water at 0C? ___ KClO3___________ e. Which substance becomes less soluble as the temperature increases? __ HCl, NH3__________ f. How would you describe the level of saturation ...
... c. Which substance is least affected by temperature change? ____ NaCl _________ d. Which substance is the least soluble in water at 0C? ___ KClO3___________ e. Which substance becomes less soluble as the temperature increases? __ HCl, NH3__________ f. How would you describe the level of saturation ...
slides
... • we present a hybrid modeling algorithm that relies on an exhaustive Smotif library and on nuclear magnetic resonance chemical shift patterns without any input of primary sequence information. • In a test of 102 proteins, the algorithm delivered 90 homologymodel-quality models, among them 24 high-q ...
... • we present a hybrid modeling algorithm that relies on an exhaustive Smotif library and on nuclear magnetic resonance chemical shift patterns without any input of primary sequence information. • In a test of 102 proteins, the algorithm delivered 90 homologymodel-quality models, among them 24 high-q ...
CHM 212 - The Federal University of Agriculture, Abeokuta
... under standard condition. It is the enthalpy change that accompanied the formation of one mole when one mole of an ionic crystal is formed from its constituent ions in the gaseous state under standard conditions. It is a measure of ionic strength. Lattice energy( ies) cannot be measured directly, bu ...
... under standard condition. It is the enthalpy change that accompanied the formation of one mole when one mole of an ionic crystal is formed from its constituent ions in the gaseous state under standard conditions. It is a measure of ionic strength. Lattice energy( ies) cannot be measured directly, bu ...
CHARMM
... CHARMM is one of the most widely used programs for computational modeling, simulations and analysis of biological (macro)molecules ...
... CHARMM is one of the most widely used programs for computational modeling, simulations and analysis of biological (macro)molecules ...
Calculations with Chemical Formulas and Equations
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined ...
Final Exam Practice Questions for General Chemistry NOTICE TO
... These exact questions will not appear on your exam. These example questions are provided to you help you review the material and become familiar with the TYPES of questions that are generally presented on the final exam. This is not a complete list of the ideas, concepts, principles, or questions co ...
... These exact questions will not appear on your exam. These example questions are provided to you help you review the material and become familiar with the TYPES of questions that are generally presented on the final exam. This is not a complete list of the ideas, concepts, principles, or questions co ...
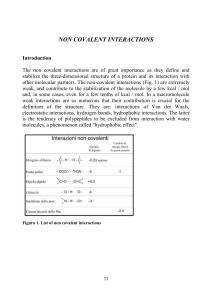
non covalent interactions
... The phenomenon is clearly described in the example in Figure 6, where it is represented an alanine alone or in the form of a dipeptide (alanine 2), tripeptide (3 alanine) or tetrapeptide (4 alanine). For each of these situations the pK relative to the protonation / deprotonation equilibrium of the C ...
... The phenomenon is clearly described in the example in Figure 6, where it is represented an alanine alone or in the form of a dipeptide (alanine 2), tripeptide (3 alanine) or tetrapeptide (4 alanine). For each of these situations the pK relative to the protonation / deprotonation equilibrium of the C ...
Polarity Notes - Embrace Challenge
... Properties of water affected by hydrogen bonding: 1. higher boiling point: Not only do molecules have to acquire enough kinetic energy to leave the liquid phase, but they need to also get enough energy to break the intermolecular forces between the water molecules. 2. capillary action: because water ...
... Properties of water affected by hydrogen bonding: 1. higher boiling point: Not only do molecules have to acquire enough kinetic energy to leave the liquid phase, but they need to also get enough energy to break the intermolecular forces between the water molecules. 2. capillary action: because water ...
structure_property
... The majority of alpha-helices in globular proteins are curved or distorted somewhat compared with the standard Pauling-Corey model. These distortions arise from several factors including: 1. The packing of buried helices against other secondary structure elements in the core of the protein. ...
... The majority of alpha-helices in globular proteins are curved or distorted somewhat compared with the standard Pauling-Corey model. These distortions arise from several factors including: 1. The packing of buried helices against other secondary structure elements in the core of the protein. ...
HERBALIFE Protein Snacks
... Berry flavors. It contains 15 grams of high-quality whey protein to help satisfy hunger and boost energy with just 70 calories. Beverage Mix comes in single-serving packets, making it convenient for on-the-go snacking. It is also available in canisters. ...
... Berry flavors. It contains 15 grams of high-quality whey protein to help satisfy hunger and boost energy with just 70 calories. Beverage Mix comes in single-serving packets, making it convenient for on-the-go snacking. It is also available in canisters. ...