lecture4
... explains the rapid nitriding of the Li wire (for CO, the two 2pp* orbitals are at -772 kJ/mol, and therefore low in energy, hence CO is a better p acceptor than N2). ...
... explains the rapid nitriding of the Li wire (for CO, the two 2pp* orbitals are at -772 kJ/mol, and therefore low in energy, hence CO is a better p acceptor than N2). ...
lecture4
... • N2 has a triple bond and hence the high dissociation energy of 940 kJ/mol. It also has a symmetrical edistribution and absence of polarity. • The lowest vacant molecular orbitals are the two degenerate antibonding p orbitals at -676 kJ, which are too high in energy to be attacked by any but the s ...
... • N2 has a triple bond and hence the high dissociation energy of 940 kJ/mol. It also has a symmetrical edistribution and absence of polarity. • The lowest vacant molecular orbitals are the two degenerate antibonding p orbitals at -676 kJ, which are too high in energy to be attacked by any but the s ...
TheMole revised
... Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. Students know one mole equals 6.02 x 1023 particles (atoms or molecules). Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic ...
... Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. Students know one mole equals 6.02 x 1023 particles (atoms or molecules). Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic ...
TG-ProteinPartners-ver8 - RI
... chemical characteristics that enable other molecules to recognize them. This activity is supported by many activities that deal with the attractions between atoms and molecules. Atomic Structure is fundamental to understanding the structure of atoms, including protons and electrons, which are essent ...
... chemical characteristics that enable other molecules to recognize them. This activity is supported by many activities that deal with the attractions between atoms and molecules. Atomic Structure is fundamental to understanding the structure of atoms, including protons and electrons, which are essent ...
STUDIES ON SURFACE PROTEINS OF
... total lysate protein and:~~rface protein, vatterns may prove useful as a biochemi~al tool for classification of Leishmaniastrains. However, ,investigations on a large number of strains that have also been characterized by other parameters are needed to clarify this possibility. The presence of cross ...
... total lysate protein and:~~rface protein, vatterns may prove useful as a biochemi~al tool for classification of Leishmaniastrains. However, ,investigations on a large number of strains that have also been characterized by other parameters are needed to clarify this possibility. The presence of cross ...
DISULFIDE GROUPS Disulfide bonds in proteins are
... applied to a column of Sephadex G-25, equilibrated with 0.1 M acetic acid. The column was developed with the same solvent. Titrations with p-mercuribenzoate (Sections 10-2 and A-2) can be used to quantitate the extent of reaction. The same procedure has also been applied to trypsinogen, chymotrypsin ...
... applied to a column of Sephadex G-25, equilibrated with 0.1 M acetic acid. The column was developed with the same solvent. Titrations with p-mercuribenzoate (Sections 10-2 and A-2) can be used to quantitate the extent of reaction. The same procedure has also been applied to trypsinogen, chymotrypsin ...
Interaction of small* molecules with membranes.
... ¾ions in solution are “point” charges ¾image effects - ignored ¾the dielectric constant is constant ...
... ¾ions in solution are “point” charges ¾image effects - ignored ¾the dielectric constant is constant ...
Document
... hydrocarbon) that had the final exam on it and it disappeared”. (Be sure to use a chemical equation, identify reactants and product(s) and include energy). ANSWER: The paper (CxHy) was burned with oxygen and the atoms in the paper are broken apart and rearranged into new combinations. The new combin ...
... hydrocarbon) that had the final exam on it and it disappeared”. (Be sure to use a chemical equation, identify reactants and product(s) and include energy). ANSWER: The paper (CxHy) was burned with oxygen and the atoms in the paper are broken apart and rearranged into new combinations. The new combin ...
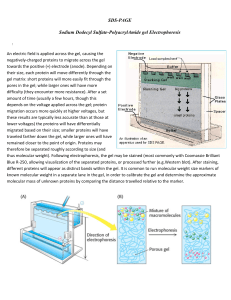
SDS-PAGE Sodium Dodecyl Sulfate
... difficulty (they encounter more resistance). After a set amount of time (usually a few hours, though this depends on the voltage applied across the gel; protein migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages) the protei ...
... difficulty (they encounter more resistance). After a set amount of time (usually a few hours, though this depends on the voltage applied across the gel; protein migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages) the protei ...
CHEM 101 Final (Term 141)
... C) The pressure at the triple point for substance A is higher than that of substance B, but the normal boiling and normal melting point for substance A are lower than those of substance B. D) The pressure at the triple point, normal boiling and normal melting point for substance B and for substance ...
... C) The pressure at the triple point for substance A is higher than that of substance B, but the normal boiling and normal melting point for substance A are lower than those of substance B. D) The pressure at the triple point, normal boiling and normal melting point for substance B and for substance ...
Chemical Formulas and Formula Weight Calculations
... of molecules which combine, and on the number of composite molecules which result. It must then be admitted that very simple relations also exist between the volumes of gaseous substances and the numbers of simple or compound molecules which form them. The first hypothesis to present itself in this ...
... of molecules which combine, and on the number of composite molecules which result. It must then be admitted that very simple relations also exist between the volumes of gaseous substances and the numbers of simple or compound molecules which form them. The first hypothesis to present itself in this ...
Chemistry Lab 2010
... • Adding a solute to a solvent decreases the freezing point ∆Tf = kf*m ∆Tf = decrease in freezing point kf = Freezing point constant (1.86 oC m-1 for water) m = molality Assumes ideal behavior and that solute is NOT ionic. How to use to find molar mass of solute: • Use ∆Tf and kf to find molality of ...
... • Adding a solute to a solvent decreases the freezing point ∆Tf = kf*m ∆Tf = decrease in freezing point kf = Freezing point constant (1.86 oC m-1 for water) m = molality Assumes ideal behavior and that solute is NOT ionic. How to use to find molar mass of solute: • Use ∆Tf and kf to find molality of ...
- University of East Anglia
... identified from their differing rotational properties in the movement between two structures representing a conformational change of a biomolecule. In DynDom3D the elements of this rotational analysis are sets of atoms within overlapping blocks placed on a grid spanning the whole molecule. The rotat ...
... identified from their differing rotational properties in the movement between two structures representing a conformational change of a biomolecule. In DynDom3D the elements of this rotational analysis are sets of atoms within overlapping blocks placed on a grid spanning the whole molecule. The rotat ...
My report on "Report Title" - RI
... chemical characteristics that enable other molecules to recognize them. This activity is supported by many activities that deal with the attractions between atoms and molecules. Atomic Structure is fundamental to understanding the structure of atoms, including protons and electrons, which are essent ...
... chemical characteristics that enable other molecules to recognize them. This activity is supported by many activities that deal with the attractions between atoms and molecules. Atomic Structure is fundamental to understanding the structure of atoms, including protons and electrons, which are essent ...
Overview of Atomic Structure and Collision Theory - OPEN-ADAS
... (antisymmetric) basis states ψν (an N -product of one-electron orbitals) with expansion coefficients aν . Spherically symmetric problem → (θ, φ) problem solved. Use standard angular algebra methods and packages are used, mostly based on Racah algebra but also Condon and Shortley (Slater-states). Onl ...
... (antisymmetric) basis states ψν (an N -product of one-electron orbitals) with expansion coefficients aν . Spherically symmetric problem → (θ, φ) problem solved. Use standard angular algebra methods and packages are used, mostly based on Racah algebra but also Condon and Shortley (Slater-states). Onl ...
“In-Gel” Digestion of Proteins in SDS-Page Gel - QB3
... We recommend Gelcode Blue® Coomassie stain (Pierce) for detecting bands. This technique works for any band that can be seen by this stain. 0.1 to 0.2 micrograms of protein is ideal. Use a new scalpel or razor blade to cut out each band. Mince each band into < 1mm2 pieces and transfer to a clean micr ...
... We recommend Gelcode Blue® Coomassie stain (Pierce) for detecting bands. This technique works for any band that can be seen by this stain. 0.1 to 0.2 micrograms of protein is ideal. Use a new scalpel or razor blade to cut out each band. Mince each band into < 1mm2 pieces and transfer to a clean micr ...
Membranlar - mustafaaltinisik.org.uk
... membrane - they are not uniformly distributed in many cases eg neurons • Lateral Asymmetry of Lipids: – Lipids can cluster in the plane of the membrane - they are not uniformly distributed – certain types may cluster around particular proteins in the membrane (a lipid entourage) – Can also induce as ...
... membrane - they are not uniformly distributed in many cases eg neurons • Lateral Asymmetry of Lipids: – Lipids can cluster in the plane of the membrane - they are not uniformly distributed – certain types may cluster around particular proteins in the membrane (a lipid entourage) – Can also induce as ...
Chapter 3 - Whitwell High School
... • When you mix the tasty pancakes, do you always make the amount that the box predicts is possible? • Or, when baking cookies…it says you can make 3 dozen do you really? Or do you eat some dough? ...
... • When you mix the tasty pancakes, do you always make the amount that the box predicts is possible? • Or, when baking cookies…it says you can make 3 dozen do you really? Or do you eat some dough? ...
Chapter 3
... • Gadolinium oxide, a colorless powder which absorbs carbon dioxide from the air, contains 86.76 mass % Gd. Determine its empirical formula. ...
... • Gadolinium oxide, a colorless powder which absorbs carbon dioxide from the air, contains 86.76 mass % Gd. Determine its empirical formula. ...
Potassium sulfate - Sigma
... For Laboratory Use Only. Not for drug, household or other uses. Preparation Instructions This product is soluble in water (66 mg/ml), yielding a clear, colorless solution. References 1. The Merck Index, 12th ed., Entry# 7845. 2. Thompson, M., et al., A comparison of the Kjeldahl and Dumas methods fo ...
... For Laboratory Use Only. Not for drug, household or other uses. Preparation Instructions This product is soluble in water (66 mg/ml), yielding a clear, colorless solution. References 1. The Merck Index, 12th ed., Entry# 7845. 2. Thompson, M., et al., A comparison of the Kjeldahl and Dumas methods fo ...
Potassium sulfate ACS Reagent Product Number - Sigma
... For Laboratory Use Only. Not for drug, household or other uses. Preparation Instructions This product is soluble in water (66 mg/ml), yielding a clear, colorless solution. References 1. The Merck Index, 12th ed., Entry# 7845. 2. Thompson, M., et al., A comparison of the Kjeldahl and Dumas methods fo ...
... For Laboratory Use Only. Not for drug, household or other uses. Preparation Instructions This product is soluble in water (66 mg/ml), yielding a clear, colorless solution. References 1. The Merck Index, 12th ed., Entry# 7845. 2. Thompson, M., et al., A comparison of the Kjeldahl and Dumas methods fo ...
Chapter 7 Chemical Formulas
... Oxyacid: contain hydrogen, oxygen and a third element. Salt: an ionic compound composed of a cation and the anion from an acid. ...
... Oxyacid: contain hydrogen, oxygen and a third element. Salt: an ionic compound composed of a cation and the anion from an acid. ...
Maple Syrup Urine Disease – Clinical Management Pathway
... MSUD Anamix infant oral or NG as tolerated, or MSUD Aid III if fluid restricted, to provide at least 3g/kg/day protein equivalent Give Isoleucine & Valine supplements, 100-200mg each, to maintain target levels (see below) If feeds poorly tolerated IV 10% dextrose + added electrolytes (+/- insulin ...
... MSUD Anamix infant oral or NG as tolerated, or MSUD Aid III if fluid restricted, to provide at least 3g/kg/day protein equivalent Give Isoleucine & Valine supplements, 100-200mg each, to maintain target levels (see below) If feeds poorly tolerated IV 10% dextrose + added electrolytes (+/- insulin ...
Review Packet - Newton.k12.ma.us
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...