Pitting Corrosion Evaluation of Titanium in NH4Br Solutions by
... The tests were performed in a three-electrode cylindrical cell using platinum as a counterelectrode and standard Ag/AgCl (3.5M KCl) as a reference electrode as shown in Fig. 1. The working electrodes were made from unalloyed titanium grade 2 with maximum content of 0.3wt% Fe, 0.1wt% C, 0.25wt% O, an ...
... The tests were performed in a three-electrode cylindrical cell using platinum as a counterelectrode and standard Ag/AgCl (3.5M KCl) as a reference electrode as shown in Fig. 1. The working electrodes were made from unalloyed titanium grade 2 with maximum content of 0.3wt% Fe, 0.1wt% C, 0.25wt% O, an ...
102MSJc14 - Louisiana Tech University
... Any chemical reaction could be considered as a forward and backward reactions occurring at the same time( ) as described previously. If the rates of backward and forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
... Any chemical reaction could be considered as a forward and backward reactions occurring at the same time( ) as described previously. If the rates of backward and forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
The interaction between colloids in polar mixtures above Tc
... sometimes other specific interactions with the polar solvent strongly depends on the solvent and the chemical nature of the ion.16–18 The total solvation energy of an ion when it is transferred from one solvent into another (the Gibbs transfer energy) is in many cases much larger than the thermal en ...
... sometimes other specific interactions with the polar solvent strongly depends on the solvent and the chemical nature of the ion.16–18 The total solvation energy of an ion when it is transferred from one solvent into another (the Gibbs transfer energy) is in many cases much larger than the thermal en ...
Fulltext PDF - Indian Academy of Sciences
... (figure 2, scheme) 9. Later this arrangement was confirmed by Chivers et al 10. The formation of the [Li3{(NtBu)3S}2]•radical is already suggested by the structure of [(thf)Li4{(NtBu)3S}2] (figure 2) in which one lithium is about to leave the area between the cap shaped dianions. When dry oxygen is ...
... (figure 2, scheme) 9. Later this arrangement was confirmed by Chivers et al 10. The formation of the [Li3{(NtBu)3S}2]•radical is already suggested by the structure of [(thf)Li4{(NtBu)3S}2] (figure 2) in which one lithium is about to leave the area between the cap shaped dianions. When dry oxygen is ...
Chapter 4: Structural features of rhenium carbene complexes
... for the equatorial carbonyl ligands of the two rhenium fragments are the same within experimental error (for [Re2(CO)10] the average eq–CO metal–carbon distance is 1.987 (6) Å). On the {Re(CO)4} fragment, that bears the carbene ligand, there are two carbonyl ligands that are trans to non–carbonyl li ...
... for the equatorial carbonyl ligands of the two rhenium fragments are the same within experimental error (for [Re2(CO)10] the average eq–CO metal–carbon distance is 1.987 (6) Å). On the {Re(CO)4} fragment, that bears the carbene ligand, there are two carbonyl ligands that are trans to non–carbonyl li ...
New interpretations of XPS spectra of nickel metal and oxides
... effect because optical and ELS experiments at that time had failed to show any structure near 6 eV and that plasmon losses could not therefore explain this satellite. We have re-examined these measurements and conclusions to relate recent ELS evidence to the interpretation of XPS core level spectra. ...
... effect because optical and ELS experiments at that time had failed to show any structure near 6 eV and that plasmon losses could not therefore explain this satellite. We have re-examined these measurements and conclusions to relate recent ELS evidence to the interpretation of XPS core level spectra. ...
Fe(H 2 O) 6 2+ + Fe(H 2 O) 6 3+ *Fe(H 2 O) 6 3+ + Fe(H 2 O) 6 2+
... magnitude slower than reduction of its conjugate base, [Co(NH3)5(OH)]3+ by Cr2+(aq). The rates of the reduction of the same cobalt complexes by [Ru(NH3)6]2+ differ by only a factor of 10. ...
... magnitude slower than reduction of its conjugate base, [Co(NH3)5(OH)]3+ by Cr2+(aq). The rates of the reduction of the same cobalt complexes by [Ru(NH3)6]2+ differ by only a factor of 10. ...
chemistry sp.indd
... There are resources for teachers and candidates written by experts. CIE also endorses a range of materials from other publishers to give a choice of approach. More information on what is available for this particular syllabus can be found at www.cie.org.uk ...
... There are resources for teachers and candidates written by experts. CIE also endorses a range of materials from other publishers to give a choice of approach. More information on what is available for this particular syllabus can be found at www.cie.org.uk ...
DFT Analysis into the Intermediates of Nickel
... protonation. Experimentally the hydride has not been isolated but is thought to form through a concerted proton coupled electron transfer (PCET) step.6 The hydride could be formed in one PCET step, or in two sequential steps, protonation and reduction.47,48 Optimized geometries for the reduced compo ...
... protonation. Experimentally the hydride has not been isolated but is thought to form through a concerted proton coupled electron transfer (PCET) step.6 The hydride could be formed in one PCET step, or in two sequential steps, protonation and reduction.47,48 Optimized geometries for the reduced compo ...
Chemistry 5325/5326 Inorganic Chemistry Spring Semester 2012
... the non-radiative rate constant increases exponentially with decreasing energy gap. For this reason, it has proven to be difficult to prepare compounds that emit in the infrared region and ...
... the non-radiative rate constant increases exponentially with decreasing energy gap. For this reason, it has proven to be difficult to prepare compounds that emit in the infrared region and ...
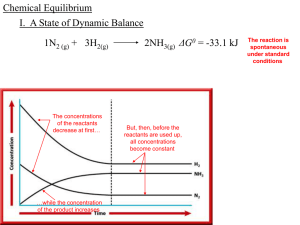
Chemical Equilibrium - local.brookings.k12.sd.us
... I. A State of Dynamic Balance -when a ________ reaction results in the almost ________ complete conversion of ________ reactants to ________, products the 1N2 (g) + 3H2(g) 2NH3(g) ________ reaction is said to go to completion but _____ most _________ reactions __________, 1N2 (g) + 3H2(g) 2NH3(g) __ ...
... I. A State of Dynamic Balance -when a ________ reaction results in the almost ________ complete conversion of ________ reactants to ________, products the 1N2 (g) + 3H2(g) 2NH3(g) ________ reaction is said to go to completion but _____ most _________ reactions __________, 1N2 (g) + 3H2(g) 2NH3(g) __ ...
chem 102 class notes - Louisiana Tech University
... Any chemical reaction could be considered as a forward and backward reactions ) as described previously. If the rates of backward and occurring at the same time( forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
... Any chemical reaction could be considered as a forward and backward reactions ) as described previously. If the rates of backward and occurring at the same time( forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
Electrolyte Solutions: Thermodynamics, Crystallization
... Phase equilibria with systems containing electrolytes are of great importance. A few examples may illustrate this: Production of fertilizers and salts is often performed by precipitation of pure solids from multi component ionic solutions. Scaling in heat exchangers is caused by some salts for which ...
... Phase equilibria with systems containing electrolytes are of great importance. A few examples may illustrate this: Production of fertilizers and salts is often performed by precipitation of pure solids from multi component ionic solutions. Scaling in heat exchangers is caused by some salts for which ...
here
... more experiments were done to test the theory. Those experiments seemed to support the idea that life, such as maggots, could be created from nonlife, such as rotting meat. All of the experiments done to confirm this theory, however, were flawed. For example, Francesco Redi, an Italian physician, sh ...
... more experiments were done to test the theory. Those experiments seemed to support the idea that life, such as maggots, could be created from nonlife, such as rotting meat. All of the experiments done to confirm this theory, however, were flawed. For example, Francesco Redi, an Italian physician, sh ...
syllabus - WordPress.com
... Since separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layeraround the colloidal particle results in a difference in potential between these layers. This potential difference between the fixed layer and the diffused layer of opp ...
... Since separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layeraround the colloidal particle results in a difference in potential between these layers. This potential difference between the fixed layer and the diffused layer of opp ...
Transition Metal Chemistry - Site title
... Crystal Field Theory • Tetrahedral ligand field • Note that ∆t = 4/9 ∆o and so ∆t is small • Therefore, tetrahedral complexes tend to blue end of spectrum energy ...
... Crystal Field Theory • Tetrahedral ligand field • Note that ∆t = 4/9 ∆o and so ∆t is small • Therefore, tetrahedral complexes tend to blue end of spectrum energy ...




![Efficient Nickel-Catalyzed [2 + 2 + 2] Cycloaddition of CO2 and Diynes](http://s1.studyres.com/store/data/020170699_1-57fd7d519966a23e70b2c51c843f4c6e-300x300.png)