Transition Metal Chemistry - Site title
... Crystal Field Theory • Tetrahedral ligand field • Note that ∆t = 4/9 ∆o and so ∆t is small • Therefore, tetrahedral complexes tend to blue end of spectrum energy ...
... Crystal Field Theory • Tetrahedral ligand field • Note that ∆t = 4/9 ∆o and so ∆t is small • Therefore, tetrahedral complexes tend to blue end of spectrum energy ...
الشريحة 1
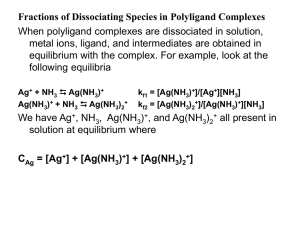
... For the case of b0, we make all terms as a function of Ag+ since b0 is a function of Ag+. We use the equilibrium constants of each step: CAg = [Ag+] + [Ag(NH3)+] + [Ag(NH3)2+] kf1 = [Ag(NH3)+]/[Ag+][NH3] [Ag(NH3)+] = kf1 [Ag+][NH3] Kf1 x kf2 = [Ag(NH3)2+]/[Ag+][NH3]2 [Ag(NH3)2+] = Kf1 x kf2 [Ag+][N ...
... For the case of b0, we make all terms as a function of Ag+ since b0 is a function of Ag+. We use the equilibrium constants of each step: CAg = [Ag+] + [Ag(NH3)+] + [Ag(NH3)2+] kf1 = [Ag(NH3)+]/[Ag+][NH3] [Ag(NH3)+] = kf1 [Ag+][NH3] Kf1 x kf2 = [Ag(NH3)2+]/[Ag+][NH3]2 [Ag(NH3)2+] = Kf1 x kf2 [Ag+][N ...
Decrease = stress More Fe(OH) 2 dissolves in response Solubility
... –smaller Ksp = less soluble –larger Ksp= more soluble ...
... –smaller Ksp = less soluble –larger Ksp= more soluble ...
Fragmentation and charge transfer in gas
... follows. First, what is the smallest dication acetonitrile complex for each metal studied; in other words, for what minimum n nmin would M2 CH3 CNn remain stable against spontaneous dissociative charge transfer? This issue gained prominence in the work on aqueous metal dications, where the exp ...
... follows. First, what is the smallest dication acetonitrile complex for each metal studied; in other words, for what minimum n nmin would M2 CH3 CNn remain stable against spontaneous dissociative charge transfer? This issue gained prominence in the work on aqueous metal dications, where the exp ...
IUPAC Provisional Recommendations
... result of two steps. In the first step the bond is cleaved but the fragments retain the structural and electronic configuration of the parent molecule; this will be followed, in the second step, by the relaxation of the fragments to their ground state. With this procedure we are considering that a b ...
... result of two steps. In the first step the bond is cleaved but the fragments retain the structural and electronic configuration of the parent molecule; this will be followed, in the second step, by the relaxation of the fragments to their ground state. With this procedure we are considering that a b ...
2 Oxidation and Oxygen Activation by Heme Proteins
... myoglobin, and the respiratory chain cytochromes have for many years ...
... myoglobin, and the respiratory chain cytochromes have for many years ...
THE ROLE OF FeSO4 IN THE OBTAINING OF
... are highly porous systems. The original porous structure providing copolymers swelling in water and high penetrability for low-molecular compounds are the main factors which allow to use them as the matrices for metal reduction [4]. Recently we have developed the blends based on PVP and 2-hydroxyeth ...
... are highly porous systems. The original porous structure providing copolymers swelling in water and high penetrability for low-molecular compounds are the main factors which allow to use them as the matrices for metal reduction [4]. Recently we have developed the blends based on PVP and 2-hydroxyeth ...
species-selective analysis for metal
... (proteases inhibitor) [29,39,40,43,45]. The homogenization step is followed by centrifugation. The use of a refrigerated ultracentrifuge (100 000 g) is strongly recommended. As a result two fractions: a soluble one (cell supernatant, cytosol) and a particulate one (cell membranes and organelles) are ...
... (proteases inhibitor) [29,39,40,43,45]. The homogenization step is followed by centrifugation. The use of a refrigerated ultracentrifuge (100 000 g) is strongly recommended. As a result two fractions: a soluble one (cell supernatant, cytosol) and a particulate one (cell membranes and organelles) are ...
Ions in crystals: The topology of the electron density in ionic
... two axial A 1 ions ~see Fig. 1!, and they play a very important role in determining the overall topology of the electronic density. After analyzing all 120 halide perovskites we have found that the electronic density can be classified into one of seven different topological schemes ~i.e., seven diff ...
... two axial A 1 ions ~see Fig. 1!, and they play a very important role in determining the overall topology of the electronic density. After analyzing all 120 halide perovskites we have found that the electronic density can be classified into one of seven different topological schemes ~i.e., seven diff ...
A discrete variable representation study of the dynamics of the
... Next step is to obtain the nuclear (vibrational) stationary states. This has been done through the use of the generic discrete variable representation (DVR) method of Colbert and Miller [19]. As small energy dierences are to be measured, the energy levels have to be obtained with high accuracy. In ...
... Next step is to obtain the nuclear (vibrational) stationary states. This has been done through the use of the generic discrete variable representation (DVR) method of Colbert and Miller [19]. As small energy dierences are to be measured, the energy levels have to be obtained with high accuracy. In ...
1 - Ankur Mittal
... (1) It is used to fill gas balloons instead of hydrogen because it is lighter and non – inflammable (2) It is used in gas – cooled nuclear reactors (3) It is used to produce and sustain powerful superconducting reagents (4) It is used as cryogenic agent for carrying out experiments at low temperatur ...
... (1) It is used to fill gas balloons instead of hydrogen because it is lighter and non – inflammable (2) It is used in gas – cooled nuclear reactors (3) It is used to produce and sustain powerful superconducting reagents (4) It is used as cryogenic agent for carrying out experiments at low temperatur ...
adsorption of zinc and copper ions on natural and ethylenediamine
... In this study, adsorption of the zinc and copper ions on montmorillonite (MMT) was investigated. In all experiments performed at pH 6.4 to 4.8, the Langmuir-Freundlich isotherm was found to best fit adsorption data that confirms a monolayer formation of the metals. The maximum amounts of the adsorbe ...
... In this study, adsorption of the zinc and copper ions on montmorillonite (MMT) was investigated. In all experiments performed at pH 6.4 to 4.8, the Langmuir-Freundlich isotherm was found to best fit adsorption data that confirms a monolayer formation of the metals. The maximum amounts of the adsorbe ...
Mimicking nitrogenase
... quiet during turnover, as well as the fact that unavoidable protons compete as oxidising reactant, frustrate experimental investigation of the chemical mechanism. A synthetic cluster with the structure of FeMo-co is not yet available.52-54 In summary, the structure of the catalytically active site ( ...
... quiet during turnover, as well as the fact that unavoidable protons compete as oxidising reactant, frustrate experimental investigation of the chemical mechanism. A synthetic cluster with the structure of FeMo-co is not yet available.52-54 In summary, the structure of the catalytically active site ( ...
Solubility and Reactions
... that the solid, liquid, or gas does not react with the solvent, water. The following list outlines how the solubility of various solutes varies with temperature. • Solids usually have higher solubility in water at higher temperatures. Figure 2 shows the solubility curves of many ionic compounds. Thi ...
... that the solid, liquid, or gas does not react with the solvent, water. The following list outlines how the solubility of various solutes varies with temperature. • Solids usually have higher solubility in water at higher temperatures. Figure 2 shows the solubility curves of many ionic compounds. Thi ...
Coordination Chemistry Reviews Metal catalysts for the vinyl
... Thus, they can be applied as a glass substitute in lenses, prisms, carrier plates and foils for optical data storage, video and compact discs. They are envisioned as cover and focusing plates for solar cells or in glass fiber optics [58]. The norbornene/ethene copolymer features a high glass-transiti ...
... Thus, they can be applied as a glass substitute in lenses, prisms, carrier plates and foils for optical data storage, video and compact discs. They are envisioned as cover and focusing plates for solar cells or in glass fiber optics [58]. The norbornene/ethene copolymer features a high glass-transiti ...
Ruthenium Olefin Metathesis Catalysts: Tuning of the Ligand Environment Ruthenium olefine
... Chapters 3, 4, 5, and 6 have been published as peer review articles. Chapter 3 is adapted from references 5,6 , chapter 4 from 7,8 , chapter 5 from 9,10 , and chapter 6 from 11 . All chapters contain their own abstract introducing the subject matter. Chapter 1 was devoted to a general introduction o ...
... Chapters 3, 4, 5, and 6 have been published as peer review articles. Chapter 3 is adapted from references 5,6 , chapter 4 from 7,8 , chapter 5 from 9,10 , and chapter 6 from 11 . All chapters contain their own abstract introducing the subject matter. Chapter 1 was devoted to a general introduction o ...