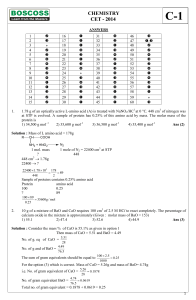
CHEMISTRY CET
... internuclear distance is equal to length of the side of the cube corresponding to volume of one CsCl ion pair. The smallest Cs to Cs internuclear distance is nearly ...
... internuclear distance is equal to length of the side of the cube corresponding to volume of one CsCl ion pair. The smallest Cs to Cs internuclear distance is nearly ...
Metal Saccharinates and Their Complexes with N
... Database (CSD) for crystal structures of saccharinates.5 In addition, we performed geometry optimizations of free saccharin molecule and free deprotonated saccharin using several semiempirical and ab initio methods.5 The April 1999 version of CSD was searched for structures that contain unsubstitute ...
... Database (CSD) for crystal structures of saccharinates.5 In addition, we performed geometry optimizations of free saccharin molecule and free deprotonated saccharin using several semiempirical and ab initio methods.5 The April 1999 version of CSD was searched for structures that contain unsubstitute ...
building bridges between inorganic and organic chemistry
... much interested in the fragments themselves as in their bonding capabilities. The moment we interact Fe(CO) 4 or CH 2 with another ligand, the initial ordering of al and b2 becomes relatively unimportant since both are typically strongly involved in the bonding. Dimerize, conceptually, the isolobal ...
... much interested in the fragments themselves as in their bonding capabilities. The moment we interact Fe(CO) 4 or CH 2 with another ligand, the initial ordering of al and b2 becomes relatively unimportant since both are typically strongly involved in the bonding. Dimerize, conceptually, the isolobal ...
picture_as_pdf Exemplars
... possible and includes a range of cognitive levels. Start by finding the written-response component. The written-response question determines the other questions that can be put on the field test so that concepts are not covered more than once. Next, look for two to three scenarios to fit your bluepr ...
... possible and includes a range of cognitive levels. Start by finding the written-response component. The written-response question determines the other questions that can be put on the field test so that concepts are not covered more than once. Next, look for two to three scenarios to fit your bluepr ...
Interactions of Metal− Metal-Bonded Antitumor Active Complexes
... activity against B-16 melanoma and sarcoma 180, respectively. Although no additional biological testings of dirhenium compounds have been reported since the studies by Eastland and co-workers,5,6 it is postulated that they inhibit DNA replication and protein synthesis similarly to dirhodium complexe ...
... activity against B-16 melanoma and sarcoma 180, respectively. Although no additional biological testings of dirhenium compounds have been reported since the studies by Eastland and co-workers,5,6 it is postulated that they inhibit DNA replication and protein synthesis similarly to dirhodium complexe ...
Mathematical Skills Handbook
... and hence the notation mol dm−3. Finding the units of a quantity may involve use of the power laws. Learners should be able to apply laws listed in Appendix A. Within the OCR GCE Chemistry qualifications, learners will in general be expected to use and recognise standard SI units. For example, dm3 i ...
... and hence the notation mol dm−3. Finding the units of a quantity may involve use of the power laws. Learners should be able to apply laws listed in Appendix A. Within the OCR GCE Chemistry qualifications, learners will in general be expected to use and recognise standard SI units. For example, dm3 i ...
(III) ion and a cobalt (II) - Iowa State University Digital Repository
... of Cr02^'^ by repeated injections of Cr^+. ...
... of Cr02^'^ by repeated injections of Cr^+. ...
Erstwhile Fyup - shivaji college
... complexes, Trans- effect, theories of trans effect, Mechanism of nucleophilic substitution in square planar complexes, Thermodynamic and Kinetic stability, Kinetics of octahedral substitution, Ligand field effects and reaction rates, Mechanism of substitution in octahedral complexes. Unit II: Introd ...
... complexes, Trans- effect, theories of trans effect, Mechanism of nucleophilic substitution in square planar complexes, Thermodynamic and Kinetic stability, Kinetics of octahedral substitution, Ligand field effects and reaction rates, Mechanism of substitution in octahedral complexes. Unit II: Introd ...
New inorganic iridium(III) complexes for photonic applications
... electrons are “circling around the nucleus”. Electrons have discrete energy levels, but the huge numbers of atoms in a solid mean that the discrete energy levels of the electrons are split into bands. The energy levels in such a band are so close together that they are almost continuous. The differe ...
... electrons are “circling around the nucleus”. Electrons have discrete energy levels, but the huge numbers of atoms in a solid mean that the discrete energy levels of the electrons are split into bands. The energy levels in such a band are so close together that they are almost continuous. The differe ...
Chapter V Binding Models for Humic Substances
... important for water quality, for chemical weathering in natural environments, and as complexing agents for trace metals. The latter property requires that humic substances are included in thermodynamic modelling of aqueous systems. However, in general thermodynamic databases, do not contain equilibr ...
... important for water quality, for chemical weathering in natural environments, and as complexing agents for trace metals. The latter property requires that humic substances are included in thermodynamic modelling of aqueous systems. However, in general thermodynamic databases, do not contain equilibr ...
Strength of the Pnicogen Bond in Complexes Involving Group Va
... electronic coupling between the local modes is eliminated. The local mode approach of Konkoli and Cremer suppresses also the kinematic coupling by starting from the mass-decoupled Euler−Lagrange equations.95 Zou and Cremer have recently demonstrated that in this way the mass-decoupled equivalent of ...
... electronic coupling between the local modes is eliminated. The local mode approach of Konkoli and Cremer suppresses also the kinematic coupling by starting from the mass-decoupled Euler−Lagrange equations.95 Zou and Cremer have recently demonstrated that in this way the mass-decoupled equivalent of ...
Non-aqueous solvent extraction of rare
... exploited to develop new, highly selective separation processes. Additionally, non-aqueous solvent extraction can be used for metal ions or metal complexes that have a strong tendency to hydrolyze in water. The solvent pairs for non-aqueous solvent extraction must fulfil the following conditions: (1 ...
... exploited to develop new, highly selective separation processes. Additionally, non-aqueous solvent extraction can be used for metal ions or metal complexes that have a strong tendency to hydrolyze in water. The solvent pairs for non-aqueous solvent extraction must fulfil the following conditions: (1 ...
1 Introduction
... molecularly dispersed in the reaction medium. Examples of homogeneous catalysts include mineral acids and transition metal compounds (e. g., rhodium carbonyl complexes in oxo synthesis). Heterogeneous catalysis takes place between several phases. Generally the catalyst is a solid, and the reactants ...
... molecularly dispersed in the reaction medium. Examples of homogeneous catalysts include mineral acids and transition metal compounds (e. g., rhodium carbonyl complexes in oxo synthesis). Heterogeneous catalysis takes place between several phases. Generally the catalyst is a solid, and the reactants ...
Coordination Chemistry and Reactivity of Monomeric Alkoxides and
... The preparation and characterization of a series of closely related magnesium and zinc compounds are reported: LMg(NiPr2)(THF), 1; LZn(NiPr2), 2; LMg(OtBu)(THF), 3; LZn(OtBu), 4; and LZn(OSiPh3)(THF), 6; where L ) CH(CMeNC6H3-2,6-iPr2)2. Their dynamic solution behavior has been examined by variable- ...
... The preparation and characterization of a series of closely related magnesium and zinc compounds are reported: LMg(NiPr2)(THF), 1; LZn(NiPr2), 2; LMg(OtBu)(THF), 3; LZn(OtBu), 4; and LZn(OSiPh3)(THF), 6; where L ) CH(CMeNC6H3-2,6-iPr2)2. Their dynamic solution behavior has been examined by variable- ...























