Ruthenium Olefin Metathesis Catalysts: Tuning of the Ligand Environment Ruthenium olefine
... Chapters 3, 4, 5, and 6 have been published as peer review articles. Chapter 3 is adapted from references 5,6 , chapter 4 from 7,8 , chapter 5 from 9,10 , and chapter 6 from 11 . All chapters contain their own abstract introducing the subject matter. Chapter 1 was devoted to a general introduction o ...
... Chapters 3, 4, 5, and 6 have been published as peer review articles. Chapter 3 is adapted from references 5,6 , chapter 4 from 7,8 , chapter 5 from 9,10 , and chapter 6 from 11 . All chapters contain their own abstract introducing the subject matter. Chapter 1 was devoted to a general introduction o ...
From concentrated metal solutions to crystalline electrides
... Alkalides - For each of the alkali metal anions, Na-, K-,Rb- and Cs- there is at least one alkalide salt of known structure in which the anions are isolated from each other. This gives us the opportunity to estimate the sizes of these anions. Since we expect them to be highly polarizable, it is like ...
... Alkalides - For each of the alkali metal anions, Na-, K-,Rb- and Cs- there is at least one alkalide salt of known structure in which the anions are isolated from each other. This gives us the opportunity to estimate the sizes of these anions. Since we expect them to be highly polarizable, it is like ...
A Chapter 4 Organometallics
... planar conformation - the metal ion must have an appropriate empty orbital for binding the H-atom MSc: f-Elements, Prof. J.-C. Bünzli, 2008 ...
... planar conformation - the metal ion must have an appropriate empty orbital for binding the H-atom MSc: f-Elements, Prof. J.-C. Bünzli, 2008 ...
GCE Getting Started - Edexcel
... elements, based on given data, in terms of structure and bonding ii. ionisation energy based on given data or recall of the plots of ionisation energy versus atomic number. Be able to illustrate periodicity using data, including electronic configurations, atomic radii, melting and boiling temperatur ...
... elements, based on given data, in terms of structure and bonding ii. ionisation energy based on given data or recall of the plots of ionisation energy versus atomic number. Be able to illustrate periodicity using data, including electronic configurations, atomic radii, melting and boiling temperatur ...
π-Arene/Cation Structure and Bonding. Solvation
... of the porphyrin cores seen in all three structures.26 As can be seen in the displacement diagrams shown in Figure 4, the particular pattern of ruffling appears to be influenced by steric effects from the arene methyl groups in the toluene and p-xylene structures. Because the ruffling leads to local ...
... of the porphyrin cores seen in all three structures.26 As can be seen in the displacement diagrams shown in Figure 4, the particular pattern of ruffling appears to be influenced by steric effects from the arene methyl groups in the toluene and p-xylene structures. Because the ruffling leads to local ...
The DNA and RNA specificity of eilatin Ru(II) complexes as
... and saturation at high DNA concentrations, suggests a simple two-state transition between the free and bound ligand (Fig. 4). By averaging the fractional change in absorbance intensity at multiple wavelengths (as a function of nucleic acid concentration), binding isotherms are generated (Figs 5 and ...
... and saturation at high DNA concentrations, suggests a simple two-state transition between the free and bound ligand (Fig. 4). By averaging the fractional change in absorbance intensity at multiple wavelengths (as a function of nucleic acid concentration), binding isotherms are generated (Figs 5 and ...
BASIC CONCEPTS OF CHEMISTRY
... of the energy of the system and its entropy, then the reaction’s direction that where the total driving force of the reaction decreases. Under the isobaric- isothermal conditions (pressure and temperature do not change) the total driving force of the reaction is called the Gibbs’ energy: G = H - T ...
... of the energy of the system and its entropy, then the reaction’s direction that where the total driving force of the reaction decreases. Under the isobaric- isothermal conditions (pressure and temperature do not change) the total driving force of the reaction is called the Gibbs’ energy: G = H - T ...
Computational Evidence of the Importance of Substituent Bulk on
... orbitals, a positive charge to increase the Lewis acidic character of the metal, numerous CH bonds to donate to the electrondeficient Ir, and no π-donor ligand to stabilize the high electron deficiency. It is thus not surprising that the crystal structure of the complex reveals two agostic bonds. Th ...
... orbitals, a positive charge to increase the Lewis acidic character of the metal, numerous CH bonds to donate to the electrondeficient Ir, and no π-donor ligand to stabilize the high electron deficiency. It is thus not surprising that the crystal structure of the complex reveals two agostic bonds. Th ...
Homogeneous Chromium Catalysts for Olefin Polymerization
... substances, most of these techniques can hardly be called definitive structural tools, and synthetic organometallic chemists would not base a detailed molecular structure solely on such results. Furthermore, the most careful studies of this type have come to the conclusion that the catalytically act ...
... substances, most of these techniques can hardly be called definitive structural tools, and synthetic organometallic chemists would not base a detailed molecular structure solely on such results. Furthermore, the most careful studies of this type have come to the conclusion that the catalytically act ...
• Both Schrock and Grubbs type alkene metathesis catalysts have a
... Mixed enyne metathesis (EneYneM) have been know to occur ...
... Mixed enyne metathesis (EneYneM) have been know to occur ...
New PSs for tackling superbugs with PDI.
... the cationic PS ensures that photogenerated singlet oxygen elicits its ascribed deleterious intracellular effect toward the microbe. This example, nicely illustrated by a Ru(II) ...
... the cationic PS ensures that photogenerated singlet oxygen elicits its ascribed deleterious intracellular effect toward the microbe. This example, nicely illustrated by a Ru(II) ...
Giant Rugby Ball [{CpBnFe(η5-P5)}24Cu96Br96
... exceeds all hitherto reported pentaphosphaferrocene-based spheres, which mostly contain 80 up to 100 framework atoms. Recently we have shown that the use of I instead of the well explored Cp* derivative [Cp*Fe(η5-P5)] allows the agglomeration of more CuX units (X = Br, I); however, the maximum numbe ...
... exceeds all hitherto reported pentaphosphaferrocene-based spheres, which mostly contain 80 up to 100 framework atoms. Recently we have shown that the use of I instead of the well explored Cp* derivative [Cp*Fe(η5-P5)] allows the agglomeration of more CuX units (X = Br, I); however, the maximum numbe ...
Proton Disorder and the Dielectric Constant of Type II Clathrate
... do not contain enough water molecules to have defects at the normal concentrations do not undergo any changes in the underlying proton structure. A simulation would remain in a single proton-disordered structure and would not sample over all the significant structures. Experimentally, the concentrat ...
... do not contain enough water molecules to have defects at the normal concentrations do not undergo any changes in the underlying proton structure. A simulation would remain in a single proton-disordered structure and would not sample over all the significant structures. Experimentally, the concentrat ...
Tunable Porosities and Shapes of FullereneLike Spheres
... used, whereas without acetonitrile, the crystallization of incomplete spheres is favored. Compounds 2 b-Br, 2 c-Br, and 2 d-Br crystallize in the same structural type and have systematically vacant inorganic scaffolds. One of the two crystallographically independent CuBr fragments is only partly occ ...
... used, whereas without acetonitrile, the crystallization of incomplete spheres is favored. Compounds 2 b-Br, 2 c-Br, and 2 d-Br crystallize in the same structural type and have systematically vacant inorganic scaffolds. One of the two crystallographically independent CuBr fragments is only partly occ ...
Metal-ligand bond lengths and strengths
... shorter bond lengths (quite in agreement with bond valence theory, where this observation is sometimes even used to calculate oxidation states [16]), but not enough to make a difference on the scale we are interested in. Comparing Cu-alcohol complexes with coordination numbers 5 and 6 it is for exam ...
... shorter bond lengths (quite in agreement with bond valence theory, where this observation is sometimes even used to calculate oxidation states [16]), but not enough to make a difference on the scale we are interested in. Comparing Cu-alcohol complexes with coordination numbers 5 and 6 it is for exam ...
Chapter 1 Review Questions
... positively or negatively charged areas. In this case, water molecules are not attracted to these substances. Instead, water molecules attract each other. Since the oil and the water remain separated (do not mix), water is, therefore, a poor solvent for oil. 27. Given the large number of manufacture ...
... positively or negatively charged areas. In this case, water molecules are not attracted to these substances. Instead, water molecules attract each other. Since the oil and the water remain separated (do not mix), water is, therefore, a poor solvent for oil. 27. Given the large number of manufacture ...
Metal-ligand bond lengths and strengths: Are they correlated? A
... shorter bond lengths (quite in agreement with bond valence theory, where this observation is sometimes even used to calculate oxidation states [16]), but not enough to make a difference on the scale we are interested in. Comparing Cu-alcohol complexes with coordination numbers 5 and 6 it is for exam ...
... shorter bond lengths (quite in agreement with bond valence theory, where this observation is sometimes even used to calculate oxidation states [16]), but not enough to make a difference on the scale we are interested in. Comparing Cu-alcohol complexes with coordination numbers 5 and 6 it is for exam ...
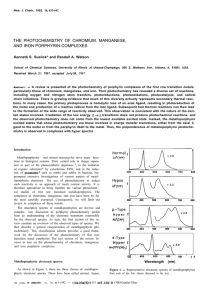
New. J. Chem., 1992, 16, 633-642
... observed and are discussed in depth in the next section of this review. In general terms, it can be said that for a redox reaction to occur there must be a second stable oxidation state available to the metal. This explains why the majority of known photoredox processes involve group 5 to 7 metals. ...
... observed and are discussed in depth in the next section of this review. In general terms, it can be said that for a redox reaction to occur there must be a second stable oxidation state available to the metal. This explains why the majority of known photoredox processes involve group 5 to 7 metals. ...
... mechanism for the electro-oxidation of formic acid at gold electrodes should include a distinct pH effect, as proposed earlier based upon results of (radio)electrochemical studies and on kinetic isotope effects [25]. The pH-dependence observed here for reaction at a Au electrode is essentially simil ...