Blue-to-green electrophosphorescence of iridium
... rings on the ligands. The mer- conformation is expected to be the kinetically favoured product from the trans dichloro-bridged dimer precursor3b since there is no bond breaking and spatial rearrangement needed. The two nitrogen atoms on the N^N ligand coordinating the Ir centre are both trans-standi ...
... rings on the ligands. The mer- conformation is expected to be the kinetically favoured product from the trans dichloro-bridged dimer precursor3b since there is no bond breaking and spatial rearrangement needed. The two nitrogen atoms on the N^N ligand coordinating the Ir centre are both trans-standi ...
General Equilibrium
... In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can be substituted for activities. (This assumption isn’t always good!) ...
... In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can be substituted for activities. (This assumption isn’t always good!) ...
Crystal field theory (II) Octahedral complexes and Jahn
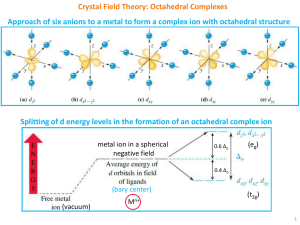
... Six ligands are considered as approaching along the three axis. So they interact more with dx2‐y2 and dz2 (eg) than with dxy, dxz and dyz orbitals (t2g). The energy of eg becomes higher and that of t2g becomes lower (degeneracy of the five orbitals is lost). The energy difference of ...
... Six ligands are considered as approaching along the three axis. So they interact more with dx2‐y2 and dz2 (eg) than with dxy, dxz and dyz orbitals (t2g). The energy of eg becomes higher and that of t2g becomes lower (degeneracy of the five orbitals is lost). The energy difference of ...
Organometallic Compounds
... – This includes interactions between the d-orbitals and the donor/-acceptor orbitals of the six ligands. – Understand this diagram in terms and strengths of the different types of interactions. – 18-electron is the most stable for this type of complex. Assuming the d-orbitals to be at similar ener ...
... – This includes interactions between the d-orbitals and the donor/-acceptor orbitals of the six ligands. – Understand this diagram in terms and strengths of the different types of interactions. – 18-electron is the most stable for this type of complex. Assuming the d-orbitals to be at similar ener ...
Formation of Inclusion Organoactinide Complexes with Boron
... Received January 8, 2004; E-mail: chmoris@tx.technion.ac.il ...
... Received January 8, 2004; E-mail: chmoris@tx.technion.ac.il ...
Ch. 15 Sections 15.6-15.8 Powerpoint
... •When placed in water, a small amount of AgCl dissolves and the following equilibrium is established once the solution becomes saturated: AgCl(s) ⇌Ag+(aq) + Cl-(aq) Equilibrium expression: If reactants or products have a coefficient other than ...
... •When placed in water, a small amount of AgCl dissolves and the following equilibrium is established once the solution becomes saturated: AgCl(s) ⇌Ag+(aq) + Cl-(aq) Equilibrium expression: If reactants or products have a coefficient other than ...
The chemistry of the transition metals
... metal followed by oxidiation state w/Roman numeral • Pt2+ = platinum (II) – If complex ion anionic, drop ending of metal • Add –ate followed by oxid. state w/Roman numeral ...
... metal followed by oxidiation state w/Roman numeral • Pt2+ = platinum (II) – If complex ion anionic, drop ending of metal • Add –ate followed by oxid. state w/Roman numeral ...
Diala , Jour , Volume , 39 , 2009
... This complex was prepared following the method describe above with the use of FeCl3.6H2O instead of MnCl2.2H2O. The violet product was filtered off , washed with EtOH and dried over P2O5 ( yield=80% ) . The chemical analyses confirmed the composition inside 0.3% error . Reagent grade chemicals were ...
... This complex was prepared following the method describe above with the use of FeCl3.6H2O instead of MnCl2.2H2O. The violet product was filtered off , washed with EtOH and dried over P2O5 ( yield=80% ) . The chemical analyses confirmed the composition inside 0.3% error . Reagent grade chemicals were ...
Ch 22 Transition complexes
... They contain atleast one Complex ion , bonded to ligands and associated with other counter ions. Coordination compound: Complex transitional metal ion attached to ligands. Two types of valance: 1. secondary Valence- Ability of metal ion to bind to a Lewis base(ligands)-Coordination number. 2. ...
... They contain atleast one Complex ion , bonded to ligands and associated with other counter ions. Coordination compound: Complex transitional metal ion attached to ligands. Two types of valance: 1. secondary Valence- Ability of metal ion to bind to a Lewis base(ligands)-Coordination number. 2. ...
File
... The dz2 and dx2y2 orbitals lie on the same axes as negative charges. Therefore, there is a large, unfavorable interaction between ligand (-) orbitals. These orbitals form the degenerate high energy pair of energy levels. The dxy , dyx and dxz orbitals bisect the negative charges. Therefore, there is ...
... The dz2 and dx2y2 orbitals lie on the same axes as negative charges. Therefore, there is a large, unfavorable interaction between ligand (-) orbitals. These orbitals form the degenerate high energy pair of energy levels. The dxy , dyx and dxz orbitals bisect the negative charges. Therefore, there is ...
1 - TAMU Chemistry
... Attempts to prepare a Kubas-type dihydrogen complex with the iridium cx{mplex shown below resulted in a 6-coordinate complex. A) Sketch the structule of the product. B) Give the oxidation states and electron counts about Ir in both reactant f>:. and product. C) Discuss why should this result be diff ...
... Attempts to prepare a Kubas-type dihydrogen complex with the iridium cx{mplex shown below resulted in a 6-coordinate complex. A) Sketch the structule of the product. B) Give the oxidation states and electron counts about Ir in both reactant f>:. and product. C) Discuss why should this result be diff ...
d-block chemistry – general considerations
... Early bonding theories: only three other atoms can attach to Co – valence of 3. Similar to FeCl3 these should be Cl- - have CoCl3 six ammonia molecules??? ...
... Early bonding theories: only three other atoms can attach to Co – valence of 3. Similar to FeCl3 these should be Cl- - have CoCl3 six ammonia molecules??? ...
Unit 10 PRACTICE Test with Answers
... 14. Identify two indicators from Reference Table M that are yellow in solutions with a pH of 5.5. any two of the following: ...
... 14. Identify two indicators from Reference Table M that are yellow in solutions with a pH of 5.5. any two of the following: ...