An 18 Electron Guideline Worksheet
... 1. First let’s figure out how to deal with charged complexes. Remember that each of the complexes, unless specified otherwise, is an 18 e- species. [Fe(CO)4]2O ...
... 1. First let’s figure out how to deal with charged complexes. Remember that each of the complexes, unless specified otherwise, is an 18 e- species. [Fe(CO)4]2O ...
Phosphine Ligands
... The increase in electron density at the nickel from PR3 σ-donation is dispersed through the M-L π system via π-backbonding. Much of the electron density is passed onto the CO π* and is reflected in decreased v(CO) stretching frequencies which corresponds to weaker CO bonds. CO stretching frequencies ...
... The increase in electron density at the nickel from PR3 σ-donation is dispersed through the M-L π system via π-backbonding. Much of the electron density is passed onto the CO π* and is reflected in decreased v(CO) stretching frequencies which corresponds to weaker CO bonds. CO stretching frequencies ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... system through the two azomethine nitrogen atoms of –C=N- and the participation of O atoms of -C=O in 2position of pyrazine ring. However, the pyridine nitrogen atom does not take part in coordination as confirmed by FTIR,UV-Visible and H NMR spectroscopy data. The newly template metal complexes hav ...
... system through the two azomethine nitrogen atoms of –C=N- and the participation of O atoms of -C=O in 2position of pyrazine ring. However, the pyridine nitrogen atom does not take part in coordination as confirmed by FTIR,UV-Visible and H NMR spectroscopy data. The newly template metal complexes hav ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... for an octahedral complex is expected to be about 4.83 B.M. (14) In a strong crystal field of octahedral symmetry (low-spin) around manganese(III) d4 ion, the 3T1g is the ground electronic state and is dominantly determined by the t2g4 electronic configuration. The magnetic moment of the spin-paired ...
... for an octahedral complex is expected to be about 4.83 B.M. (14) In a strong crystal field of octahedral symmetry (low-spin) around manganese(III) d4 ion, the 3T1g is the ground electronic state and is dominantly determined by the t2g4 electronic configuration. The magnetic moment of the spin-paired ...
Nature of Acids and Bases
... ionization of water, which of the following is true? (1) The forward reaction forming ions from water is favored. (2) The concentration of ions in pure water is high. (3) The concentration of hydronium in pure water is higher than the concentration of hydroxide. (4) The concentration of ions in pure ...
... ionization of water, which of the following is true? (1) The forward reaction forming ions from water is favored. (2) The concentration of ions in pure water is high. (3) The concentration of hydronium in pure water is higher than the concentration of hydroxide. (4) The concentration of ions in pure ...
science background - CMA
... base "soaks up" hydrogen ions, the result is a solution with more hydroxide ions than hydrogen ions. This kind of solution is basic (alkaline). ...
... base "soaks up" hydrogen ions, the result is a solution with more hydroxide ions than hydrogen ions. This kind of solution is basic (alkaline). ...
Synthesis and structure of copper(II) complexes: Potential cyanide
... matrix least square refinements on F2 were performed using SHELXL-97 program.63 All the non-hydrogen atoms were refined anisotropically using full-matrix least squares method. Hydrogen atoms were included for structure factor calculations after placing them at calculated positions. Atomic coordinates ...
... matrix least square refinements on F2 were performed using SHELXL-97 program.63 All the non-hydrogen atoms were refined anisotropically using full-matrix least squares method. Hydrogen atoms were included for structure factor calculations after placing them at calculated positions. Atomic coordinates ...
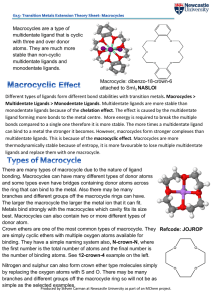
Different types of ligands form different bond stabilities with transition
... monodentate ligands because of the chelation effect. The effect is caused by the multidentate ligand forming more bonds to the metal centre. More energy is required to break the multiple bonds compared to a single one therefore it is more stable. The more times a multidentate ligand can bind to a me ...
... monodentate ligands because of the chelation effect. The effect is caused by the multidentate ligand forming more bonds to the metal centre. More energy is required to break the multiple bonds compared to a single one therefore it is more stable. The more times a multidentate ligand can bind to a me ...
Isolation of Cobalt (II) and Copper (II)
... potentials. In some of these works, the changes in free energy of coordination bonds as a result of mixed ligand complex formation has been considered. Studies have been carried out on mixed complexes of nickel (II) with only amino, carboxylate and water as the coordinate groups. The ligands were ch ...
... potentials. In some of these works, the changes in free energy of coordination bonds as a result of mixed ligand complex formation has been considered. Studies have been carried out on mixed complexes of nickel (II) with only amino, carboxylate and water as the coordinate groups. The ligands were ch ...
B H B H - B H 2- B H 2-
... reactions of metalloboranes is reviewed. In the decaborane series, single—crystal X-ray studies indicate that the metal atom can be (a) fully incorporated within the borane cluster, (b) joined to the cluster by an exopolyhedral B-M-B bond in which the metal replaces a bridging proton, or (c) joined ...
... reactions of metalloboranes is reviewed. In the decaborane series, single—crystal X-ray studies indicate that the metal atom can be (a) fully incorporated within the borane cluster, (b) joined to the cluster by an exopolyhedral B-M-B bond in which the metal replaces a bridging proton, or (c) joined ...
Quimica Coordinacion IV Mecanismos de reaccion Cap 12 Miessler
... Images from Miessler and Tarr “Inorganic Chemistry” 2011 obtained from Pearson Education, Inc. ...
... Images from Miessler and Tarr “Inorganic Chemistry” 2011 obtained from Pearson Education, Inc. ...
Test 4 Review - Ralph C. Mahar
... What mass of copper will be deposited by a current of 7.89 amps flowing for 1200 seconds? Cu2+ + 2e- g Cu at the cathode 7.89A x 1200s x 1C x 1 mol e- = .0981 mol eA·s 96,485C ...
... What mass of copper will be deposited by a current of 7.89 amps flowing for 1200 seconds? Cu2+ + 2e- g Cu at the cathode 7.89A x 1200s x 1C x 1 mol e- = .0981 mol eA·s 96,485C ...
Chemistry MSL Practical Style Review 1. What is the nuclear
... Delocalized electrons move among many atoms creating a sea of electrons. ...
... Delocalized electrons move among many atoms creating a sea of electrons. ...
Wine Country Lodging near San Luis Obispo CA
... as the EAN rule, that in a number of metal complexes the metal atom tends to surround itself with sufficient ligands that the resul�ng effec�ve atomic number is numerically equal to the atomic number ...
... as the EAN rule, that in a number of metal complexes the metal atom tends to surround itself with sufficient ligands that the resul�ng effec�ve atomic number is numerically equal to the atomic number ...
1 Q. If ΔrH is positive, what can you say about the reaction? 2 Q If
... 16 Q. What is the value of [C] in the equilibrium constant expression for the reaction and what is Kc: C(s) + O2(g) Ù CO2(g)? ...
... 16 Q. What is the value of [C] in the equilibrium constant expression for the reaction and what is Kc: C(s) + O2(g) Ù CO2(g)? ...
Some chemistry of the Periodic Table Electronic configuration and
... There are certain rules for assigning and using oxidation numbers. These are: 1. The oxidation number in a free or uncombined element is zero. Thus, metallic magnesium has an oxidation number of zero as does Chlorine in chlorine gas, Cl2. 2. For ions consisting of single atoms the oxidation number i ...
... There are certain rules for assigning and using oxidation numbers. These are: 1. The oxidation number in a free or uncombined element is zero. Thus, metallic magnesium has an oxidation number of zero as does Chlorine in chlorine gas, Cl2. 2. For ions consisting of single atoms the oxidation number i ...
Discussion questions for Quintuple Bond Paper
... O2 . The O–O bond in the superoxo ligand should be slightly stronger (higher bond order) and shorter than in the peroxo ligand. X-ray diffraction studies could provide a measure of the O–O bond distance in the coordinated ligand and this could be compared to other bond distances from known superoxid ...
... O2 . The O–O bond in the superoxo ligand should be slightly stronger (higher bond order) and shorter than in the peroxo ligand. X-ray diffraction studies could provide a measure of the O–O bond distance in the coordinated ligand and this could be compared to other bond distances from known superoxid ...
communication - Durham Research Online
... The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-pro t purposes provided that: • a full bibliographic reference is made to the original source • a link is made ...
... The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-pro t purposes provided that: • a full bibliographic reference is made to the original source • a link is made ...
МІНІСТЕРСТВО ОХОРОНИ ЗДОРОВ`Я УКРАЇНИ ХАРКІВСЬКИЙ
... Acidocomplexes of platinum (II): [Pt(NH3)4]Cl2, [Pt(NH3)3Cl]Cl, [Pt(NH3)2Cl2], [Pt(NH3)Cl3], and K2[PtCl4]. Cyclic or chelate (from the Greek word «chele» – claw) complex compounds contain a bi- or polydentate ligand that grips the central atom, as it were, like the claws of a crab. Chelating ligan ...
... Acidocomplexes of platinum (II): [Pt(NH3)4]Cl2, [Pt(NH3)3Cl]Cl, [Pt(NH3)2Cl2], [Pt(NH3)Cl3], and K2[PtCl4]. Cyclic or chelate (from the Greek word «chele» – claw) complex compounds contain a bi- or polydentate ligand that grips the central atom, as it were, like the claws of a crab. Chelating ligan ...
1 NEUTRON DIFFRACTION STUDIES OF METAL
... Hydrogen atoms have the ability to occupy interstitial cavities in metal lattices. It is well known that hydrogen can occupy interstitial sites in non-stoichiometric hydrides of metals leading to high coordination [9]. These materials have historically been studied with surface diffraction technique ...
... Hydrogen atoms have the ability to occupy interstitial cavities in metal lattices. It is well known that hydrogen can occupy interstitial sites in non-stoichiometric hydrides of metals leading to high coordination [9]. These materials have historically been studied with surface diffraction technique ...