Fe superoxide dismutase - Chemistry
... His30 is absolutely conserved in FeSODs and MnSODs and the mutation of His30 in human MnSOD decreases kcat and can increase KM71 (but see reference 72). If F2 were to associate with Fe2+SOD in the Ê from Fe2+. place of this solvent molecule, it would be 7.5 A However this distance should be regarded ...
... His30 is absolutely conserved in FeSODs and MnSODs and the mutation of His30 in human MnSOD decreases kcat and can increase KM71 (but see reference 72). If F2 were to associate with Fe2+SOD in the Ê from Fe2+. place of this solvent molecule, it would be 7.5 A However this distance should be regarded ...
A high-field solid-state Cl NMR and quantum chemical
... A series of six L-amino acid hydrochloride salts has been studied by 35/37Cl solid-state NMR spectroscopy (at 11.75 and 21.1 T) and complementary quantum chemical calculations. Analyses of NMR spectra acquired under static and magic-angle-spinning conditions for the six hydrochloride salts, those of ...
... A series of six L-amino acid hydrochloride salts has been studied by 35/37Cl solid-state NMR spectroscopy (at 11.75 and 21.1 T) and complementary quantum chemical calculations. Analyses of NMR spectra acquired under static and magic-angle-spinning conditions for the six hydrochloride salts, those of ...
Insulators
... prove the total incorporation of In3+ and Co2+ ions in the lattice of the synthesised solid precursors. Also, the XRD data reveal that the prepared CdCl2.H2O precursors were nanocrystallite powders. The XRD of final synthesised powders are shown in fig.3. The patterns reveal that the synthesised po ...
... prove the total incorporation of In3+ and Co2+ ions in the lattice of the synthesised solid precursors. Also, the XRD data reveal that the prepared CdCl2.H2O precursors were nanocrystallite powders. The XRD of final synthesised powders are shown in fig.3. The patterns reveal that the synthesised po ...
Correlated/non-correlated ion dynamics of charge
... The title salt, 3-butyl-1-methyl-1H-imidazolium hexafluorophosphate, [C4mim][PF6] (see Fig. 1), has to date received much attention in the arena of molecular dynamics (MD), usually employing static high field approaches that probe fast motions.9–11 Our interest in this particular salt, which possess ...
... The title salt, 3-butyl-1-methyl-1H-imidazolium hexafluorophosphate, [C4mim][PF6] (see Fig. 1), has to date received much attention in the arena of molecular dynamics (MD), usually employing static high field approaches that probe fast motions.9–11 Our interest in this particular salt, which possess ...
Palladium-Catalyzed Synthesis and Transformation of Organoboranes
... amounts of Pd2(dba)3. By employment of commercially available chiral diboronates enantioenriched homoallyl alcohols could be obtained. We have also developed a palladium-catalyzed method for synthesis of functionalized allylboronic acids from vinyl cyclopropane, vinyl aziridine, allyl acetate and al ...
... amounts of Pd2(dba)3. By employment of commercially available chiral diboronates enantioenriched homoallyl alcohols could be obtained. We have also developed a palladium-catalyzed method for synthesis of functionalized allylboronic acids from vinyl cyclopropane, vinyl aziridine, allyl acetate and al ...
Chapter 2 1.Certain gases in the 293K and 9.97 × 104Pa when the
... Why do some non-metallic elements (such as F, O, etc.) have become abnormal? Answer: In general, the electron affinity and energy with the decrease of atomic radius increases because the radius of hours, the nuclear charge of the electron cited large, therefore, electron affinity and energy left to ...
... Why do some non-metallic elements (such as F, O, etc.) have become abnormal? Answer: In general, the electron affinity and energy with the decrease of atomic radius increases because the radius of hours, the nuclear charge of the electron cited large, therefore, electron affinity and energy left to ...
Study Guide--Ch. 1-3 - Cedarville University
... Be able to identify the particles present in solution when an acid or a base is dissolved in water. Table 10.1 contains a list of strong acids and bases. Know the name and formula for each of the substances. Be able to identify the most common strong and weak acids and bases. Be able to identify the ...
... Be able to identify the particles present in solution when an acid or a base is dissolved in water. Table 10.1 contains a list of strong acids and bases. Know the name and formula for each of the substances. Be able to identify the most common strong and weak acids and bases. Be able to identify the ...
Physical Chemistry
... thermodynamic properties namely internal energy (U), enthalpy (H), entropy (S) and free energy functions (A and G) and their variations with variables like temperature, pressure, volume and amount. The changes in these properties depend only on the initial and final states of the system, and are ind ...
... thermodynamic properties namely internal energy (U), enthalpy (H), entropy (S) and free energy functions (A and G) and their variations with variables like temperature, pressure, volume and amount. The changes in these properties depend only on the initial and final states of the system, and are ind ...
Solving General Chemistry Problems 5e
... only arithmetic and simple algebra. Nevertheless, if you don't understand it, you can expect troubles before long. So, before you can really get into chemistry, you need to master the mathematical operations in the first six chapters. 3. Don't think of your calculator as a security blanket that will ...
... only arithmetic and simple algebra. Nevertheless, if you don't understand it, you can expect troubles before long. So, before you can really get into chemistry, you need to master the mathematical operations in the first six chapters. 3. Don't think of your calculator as a security blanket that will ...
Experiments in General Chemistry: Featuring MeasureNet
... Chemists observe matter by determining, measuring, and monitoring physical and chemical properties of matter. A property is any characteristic that can be used to describe matter (e.g., size, color, mass, density, solubility, etc.). In this experiment, we will determine the density of liquids and so ...
... Chemists observe matter by determining, measuring, and monitoring physical and chemical properties of matter. A property is any characteristic that can be used to describe matter (e.g., size, color, mass, density, solubility, etc.). In this experiment, we will determine the density of liquids and so ...
Vol 1 No 2.10
... decreases linearly, and the concentration exponentially. The flux equation expressed by the Eq.(2) is similar to Ohm’s and Fourier’s law not only in the form of mathematical formulations, but also in physical significance of the terms. Recently Islam [2] made a controversial conclusion that the both ...
... decreases linearly, and the concentration exponentially. The flux equation expressed by the Eq.(2) is similar to Ohm’s and Fourier’s law not only in the form of mathematical formulations, but also in physical significance of the terms. Recently Islam [2] made a controversial conclusion that the both ...
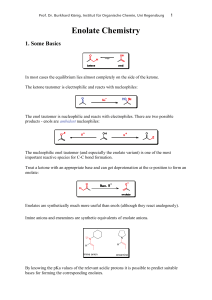
Enolate Chemistry - Institut für Organische Chemie
... Prof. Dr. Burkhard König, Institut für Organische Chemie, Uni Regensburg ...
... Prof. Dr. Burkhard König, Institut für Organische Chemie, Uni Regensburg ...
WH n under pressure
... An initial observation of the formation of WH under pressure from W gaskets surrounding hydrogen in diamond anvil cells led to a theoretical study of tungsten hydride phases. At P = 1 atm no stoichiometry is found to be stable with respect to separation into the elements, but as the pressure is rais ...
... An initial observation of the formation of WH under pressure from W gaskets surrounding hydrogen in diamond anvil cells led to a theoretical study of tungsten hydride phases. At P = 1 atm no stoichiometry is found to be stable with respect to separation into the elements, but as the pressure is rais ...
10. Solution Guide to Supplementary Exercises
... In experiment A, 1.00 mol dm–3 H2(g) and 1.00 mol dm–3 I2(g) were initially added to a flask and equilibrium was established. In experiment B, 2.00 mol dm–3 HI(g) were initially added to a second flask and equilibrium was established. Which of the following statements is always true about the equili ...
... In experiment A, 1.00 mol dm–3 H2(g) and 1.00 mol dm–3 I2(g) were initially added to a flask and equilibrium was established. In experiment B, 2.00 mol dm–3 HI(g) were initially added to a second flask and equilibrium was established. Which of the following statements is always true about the equili ...
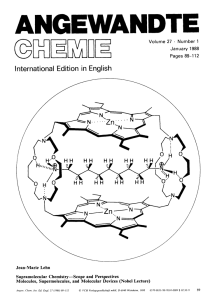
Supramolecular Chemistry—Scope and Perspectives Molecules
... coordination. Supramolecular catalysis by receptors bearing reactive groups effects bond cleavage reactions as well as synthetic bond formation via cocatalysis. Lipophilic receptor molecules act as selective carriers for various substrates and make it possible to set up coupled transport processes l ...
... coordination. Supramolecular catalysis by receptors bearing reactive groups effects bond cleavage reactions as well as synthetic bond formation via cocatalysis. Lipophilic receptor molecules act as selective carriers for various substrates and make it possible to set up coupled transport processes l ...
György Dombi Gerda Szakonyi Authors
... Pharmacopoeia 8th Edition, the maximum allowed conductance of Aqua purificata is 4.3 μS/cm, while that of Aqua ad iniectabilia is 1.1 μS/cm. Conductometric titrations can be applied when the ion concentration changes during a reaction, or when the ion concentration remains constant, but the mobility ...
... Pharmacopoeia 8th Edition, the maximum allowed conductance of Aqua purificata is 4.3 μS/cm, while that of Aqua ad iniectabilia is 1.1 μS/cm. Conductometric titrations can be applied when the ion concentration changes during a reaction, or when the ion concentration remains constant, but the mobility ...
Iridium Complex-Catalyzed Highly Selective Organic Synthesis
... An iridium complex was found to be an efficient catalyst for highly branched product-selective allylic alkylation.16 We examined the reaction of (E)-2-hexenyl acetate [(E)2a] with diethyl sodiomalonate (3a) in the presence of a catalytic amount of [Ir(cod)Cl]2 (Ir atom 4 mol%), and found that an add ...
... An iridium complex was found to be an efficient catalyst for highly branched product-selective allylic alkylation.16 We examined the reaction of (E)-2-hexenyl acetate [(E)2a] with diethyl sodiomalonate (3a) in the presence of a catalytic amount of [Ir(cod)Cl]2 (Ir atom 4 mol%), and found that an add ...
AS Chemistry Teacher Handbook
... Candidates should know that 12C is used as the standard in comparing relative masses. Candidates should be able to use relative atomic masses to calculate relative formula masses. Candidates will not be expected to draw a diagram of ...
... Candidates should know that 12C is used as the standard in comparing relative masses. Candidates should be able to use relative atomic masses to calculate relative formula masses. Candidates will not be expected to draw a diagram of ...
Lecture 06 Slides
... Rules for determining the oxidation number of an element within a compound Step 1: Write the oxidation number of each known atom below the atom in the formula Step 2: Multiply each oxidation number by the number of atoms of that element in the compound Step 3: Assign oxidation numbers for the other ...
... Rules for determining the oxidation number of an element within a compound Step 1: Write the oxidation number of each known atom below the atom in the formula Step 2: Multiply each oxidation number by the number of atoms of that element in the compound Step 3: Assign oxidation numbers for the other ...
An experimentally validated numerical model of interface advance of
... water vapor pressure is constant. However, as pointed out by Modestov et al. [18], the size of samples used in those studies is comparable to the size of the reaction zone itself, which is insufficient to draw a conclusion of constant propagation rate. Therefore, experiments on the propagation of th ...
... water vapor pressure is constant. However, as pointed out by Modestov et al. [18], the size of samples used in those studies is comparable to the size of the reaction zone itself, which is insufficient to draw a conclusion of constant propagation rate. Therefore, experiments on the propagation of th ...
Energetics
... that the total enthalpy change accompanying a chemical reaction is independent of the route by which the chemical reaction takes place and depends only on the difference between the total enthalpy of the reactants and that of the products. ...
... that the total enthalpy change accompanying a chemical reaction is independent of the route by which the chemical reaction takes place and depends only on the difference between the total enthalpy of the reactants and that of the products. ...