Article - Max Planck Institut für Festkörperforschung
... properties.[3] On the other hand, the exact number of such heteroatoms is extremely difficult to control. This ªchemistry gapº also becomes evident for very large molecular entities with a certain number of functional groups. The best examples are probably proteins where a single amino acid change c ...
... properties.[3] On the other hand, the exact number of such heteroatoms is extremely difficult to control. This ªchemistry gapº also becomes evident for very large molecular entities with a certain number of functional groups. The best examples are probably proteins where a single amino acid change c ...
TRIPURA UNIVERSITY SURYAMANINAGAR SYLLABUS FOR B.Sc THREE-YEAR DEGREE (GENERAL AND HONOURS) COURSE
... formation and reactivity application of Grignard reagents in organic synthesis and its limitations. (iii) Organic Compounds Containing nitrogen (aliphatic): Methods of synthesis and reactions of nitroalkanes, alkyl nitrites, alkyl cyanides, isocyanides. Introduction to amino compounds, general metho ...
... formation and reactivity application of Grignard reagents in organic synthesis and its limitations. (iii) Organic Compounds Containing nitrogen (aliphatic): Methods of synthesis and reactions of nitroalkanes, alkyl nitrites, alkyl cyanides, isocyanides. Introduction to amino compounds, general metho ...
Interaction of the Adenine-Thymine Watson
... Quantum chemical calculations have been used for a long time to investigate nucleic acid base pairs1 and their complexes with metal ions.2 Most of these calculations2 applied (at best) the Hartree-Fock approximation with a minimal basis set of atomic orbitals. The gradient geometry optimization was ...
... Quantum chemical calculations have been used for a long time to investigate nucleic acid base pairs1 and their complexes with metal ions.2 Most of these calculations2 applied (at best) the Hartree-Fock approximation with a minimal basis set of atomic orbitals. The gradient geometry optimization was ...
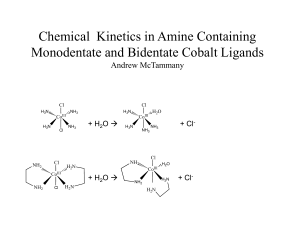
Chemical Kinetics in Monodentate and Bidentate Cobalt Compounds
... repeated. The trans-Co(NH3)4Cl2 should be synthesized using a lower temperature and lower concentration of acid. A mixture of HCl and H2SO4 could be used instead. From there the effect of different ligands can be evaluated. ...
... repeated. The trans-Co(NH3)4Cl2 should be synthesized using a lower temperature and lower concentration of acid. A mixture of HCl and H2SO4 could be used instead. From there the effect of different ligands can be evaluated. ...
Chemistry of the d
... e.g.: i) all Co(III) Complexes are diamagnetic; except [CoF6]3-, [CoF3(H2O)3] ii) Cr(III) in Oh: 3 unpaired e−; Cr(II) in Oh: predicted: 2e−, exp.: 4 unpaired e− 2) Ni(II) planar vs. octahedral is supposed to introduce change from covalent to ionic e.g.: [Ni(en)2]2+ 2NH3 Æ [Ni(NH3)2(en)2] 3) Two Ato ...
... e.g.: i) all Co(III) Complexes are diamagnetic; except [CoF6]3-, [CoF3(H2O)3] ii) Cr(III) in Oh: 3 unpaired e−; Cr(II) in Oh: predicted: 2e−, exp.: 4 unpaired e− 2) Ni(II) planar vs. octahedral is supposed to introduce change from covalent to ionic e.g.: [Ni(en)2]2+ 2NH3 Æ [Ni(NH3)2(en)2] 3) Two Ato ...
Polyhedral Oligomeric Silsesquioxane
... layer-by-layer assembly onto an organic solid support.23 However, being a soft material, the ligand/polymer may not provide enough robustness against metal leaching or for recyclability.24 In recent years, the ionic liquids and the related solid ionic liquid phase catalysis have been successfully us ...
... layer-by-layer assembly onto an organic solid support.23 However, being a soft material, the ligand/polymer may not provide enough robustness against metal leaching or for recyclability.24 In recent years, the ionic liquids and the related solid ionic liquid phase catalysis have been successfully us ...
fundamental concept of transition and inner
... Saharanpur for his constant inspiration, intuitive guidance and timely help throughout the phase of my work. I am thankful to Dr. Vijai Malik, Assistant Professor, Department of Botany, Maharaj Singh College Saharanpur for his constant guidance and motivation for writing this book. I extend my thank ...
... Saharanpur for his constant inspiration, intuitive guidance and timely help throughout the phase of my work. I am thankful to Dr. Vijai Malik, Assistant Professor, Department of Botany, Maharaj Singh College Saharanpur for his constant guidance and motivation for writing this book. I extend my thank ...
Introduction
... complexation of trivalent 5f over 4f ions, finding a particular application in the reprocessing of spent nuclear fuel.11 Since the bonds between the BH4 ligand and d transition metals or felements are reputed to have a significant covalent character, it was appealing to determine if, and by what amo ...
... complexation of trivalent 5f over 4f ions, finding a particular application in the reprocessing of spent nuclear fuel.11 Since the bonds between the BH4 ligand and d transition metals or felements are reputed to have a significant covalent character, it was appealing to determine if, and by what amo ...
Characterization of Low Energy Charge Transfer Transitions in
... were determined with a Shimadzu UV-3101PC or UV-2101PC spectrophotometer at 298 K. Deconvolutions of the lowest energy observed MLCT absorption bands were performed using the multipeak 2.0 routine in the IGOR Pro 6.2.1.0 program.31 The observed absorption spectra, Iobsd, have been ...
... were determined with a Shimadzu UV-3101PC or UV-2101PC spectrophotometer at 298 K. Deconvolutions of the lowest energy observed MLCT absorption bands were performed using the multipeak 2.0 routine in the IGOR Pro 6.2.1.0 program.31 The observed absorption spectra, Iobsd, have been ...
THE d- AND f-BLOCK ELEMENTS
... available on them hence they can accept lone pairs of electrons forming coordinate covalent bond. The greater the charge density on the transition metal ion, the greater they have tendency to form complexes. Thus Ti+2 to Ni+2 the stability of complexes formed goes on increasing. Compounds like NaCl, ...
... available on them hence they can accept lone pairs of electrons forming coordinate covalent bond. The greater the charge density on the transition metal ion, the greater they have tendency to form complexes. Thus Ti+2 to Ni+2 the stability of complexes formed goes on increasing. Compounds like NaCl, ...
Detection of Cysteine with Glutathione-capped
... and -COO- groups. Upon the addition of acid, the carboxylic acid groups are protonated. This means that in the gly part of GSH, the interaction between the carboxylate and the gold surface is lost upon protonation and only the -SH interaction remains. Basu et al. [33] and Lim et al. [34] supported t ...
... and -COO- groups. Upon the addition of acid, the carboxylic acid groups are protonated. This means that in the gly part of GSH, the interaction between the carboxylate and the gold surface is lost upon protonation and only the -SH interaction remains. Basu et al. [33] and Lim et al. [34] supported t ...
Grade XII Unit 1 - Ethiopian Ministry of Education
... Ethanol mixes with water but oil does not. Why? Solubility is a measure of how much solute will dissolve in a solvent at a specific temperature. Do you know the principle “like dissolves like”? The “like dissolves like” principle is helpful in predicting the solubility of a substance in a given solv ...
... Ethanol mixes with water but oil does not. Why? Solubility is a measure of how much solute will dissolve in a solvent at a specific temperature. Do you know the principle “like dissolves like”? The “like dissolves like” principle is helpful in predicting the solubility of a substance in a given solv ...
Dodonaea viscosa Steel – A Green approach S. Leelavathi, R. Rajalakshmi
... HCl and 0.5M H2SO4 solutions at room temperature at different periods of immersion are presented in table 1. From the table, corrosion rate decreases noticeably with an increase in DVLE concentration, i.e. the corrosion inhibition enhances with the inhibitor concentration. This behavior is due to th ...
... HCl and 0.5M H2SO4 solutions at room temperature at different periods of immersion are presented in table 1. From the table, corrosion rate decreases noticeably with an increase in DVLE concentration, i.e. the corrosion inhibition enhances with the inhibitor concentration. This behavior is due to th ...
AQA A-level Chemistry
... than the reactant line as the reaction has absorbed energy. Conversely, in an exothermic reaction the product line is at a lower enthalpy value than the reactant line as the reaction has released energy. The difference between the lines is labelled ΔH (change in enthalpy). In an endothermic reaction ...
... than the reactant line as the reaction has absorbed energy. Conversely, in an exothermic reaction the product line is at a lower enthalpy value than the reactant line as the reaction has released energy. The difference between the lines is labelled ΔH (change in enthalpy). In an endothermic reaction ...
Higher-Coordinated Molecular Silicon Compounds
... selected nitridosilicates, and so-called sialons (silicon aluminum nitride oxides), which contain sixfold-coordinated silicon atoms, have been reported [8–10]. An even larger variety of molecular compounds containing higher-coordinated silicon atoms are known. These include neutral, anionic, as well ...
... selected nitridosilicates, and so-called sialons (silicon aluminum nitride oxides), which contain sixfold-coordinated silicon atoms, have been reported [8–10]. An even larger variety of molecular compounds containing higher-coordinated silicon atoms are known. These include neutral, anionic, as well ...
ANNEX (Manuscrits posteriors a la Comissió de Doctorat de Juliol del...
... or amines,7 phenolate, dialkyl or diarylphosphite8 and Nalkylcarbamoyldiphenylphosphine oxides9 resulting in one anionic species as a consequence of the opening of the dioxane ring. But mono or di B(8)-R (R= alkyl) substituted derivatives of [1]- have not yet been reported. In this work we report on ...
... or amines,7 phenolate, dialkyl or diarylphosphite8 and Nalkylcarbamoyldiphenylphosphine oxides9 resulting in one anionic species as a consequence of the opening of the dioxane ring. But mono or di B(8)-R (R= alkyl) substituted derivatives of [1]- have not yet been reported. In this work we report on ...
Structurally simple
... The formation of a strong covalent bond between the Lewis acidic carbon atom of CO2 and a donor atom of a Lewis base leads to the most common examples of CO2 complexes found in the literature. Carbamates, formed through the reaction of CO2 with amines, an interaction taken advantage of in the field ...
... The formation of a strong covalent bond between the Lewis acidic carbon atom of CO2 and a donor atom of a Lewis base leads to the most common examples of CO2 complexes found in the literature. Carbamates, formed through the reaction of CO2 with amines, an interaction taken advantage of in the field ...
sol-gel chemistry of transition metal oxides
... each atom acquires a positive or negative partial charge 6 i . It is usually assumed that the electronegativity Xi of an atom changes linearly with its charge 38 ...
... each atom acquires a positive or negative partial charge 6 i . It is usually assumed that the electronegativity Xi of an atom changes linearly with its charge 38 ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... g/dm3). However, these values are all below -20 kJ/mol. The interaction can, therefore, be said to more or less weak electrostatic interactions as the energy values are of the range of the van der Waal, London forces, dipole-dipole attractions, etc. which are actually of weak forces [14, 21, 22]. As ...
... g/dm3). However, these values are all below -20 kJ/mol. The interaction can, therefore, be said to more or less weak electrostatic interactions as the energy values are of the range of the van der Waal, London forces, dipole-dipole attractions, etc. which are actually of weak forces [14, 21, 22]. As ...