Formation Mechanisms of Naphthalene and
... 3.1. Crossed Molecular Beam Setup. The crossed molecular beam technique represents an unprecedented approach to reveal the outcome of a reaction of two neutral molecules, radicals, and/or atoms in the single collision environment without wall effects.16−19 This is achieved by generating supersonic be ...
... 3.1. Crossed Molecular Beam Setup. The crossed molecular beam technique represents an unprecedented approach to reveal the outcome of a reaction of two neutral molecules, radicals, and/or atoms in the single collision environment without wall effects.16−19 This is achieved by generating supersonic be ...
Simple, Fast and Selective Detection of Adenosine Triphosphate at
... As shown in Figure 2, the AuNPs were red in color and exhibited an absorption peak at 520 nm (A520), which was ascribed to its surface plasmon resonance (vial 1 and black curve). No obvious change was observed upon the addition of ATP (vial 2) or Cu2+ (vial 3) alone. However, when ATP and Cu2+ succe ...
... As shown in Figure 2, the AuNPs were red in color and exhibited an absorption peak at 520 nm (A520), which was ascribed to its surface plasmon resonance (vial 1 and black curve). No obvious change was observed upon the addition of ATP (vial 2) or Cu2+ (vial 3) alone. However, when ATP and Cu2+ succe ...
BSC HS CHEMISTRY SEMESTER I to VI CBCEGS
... Students will be required to read a prescribed prose anthology titled Selections from Modern English Prose (Ed. Haladhar Panda published by University Press, Hyderabad). The essays in the anthology will be read by students at home with the help of glossary given in the book. Progressing from one les ...
... Students will be required to read a prescribed prose anthology titled Selections from Modern English Prose (Ed. Haladhar Panda published by University Press, Hyderabad). The essays in the anthology will be read by students at home with the help of glossary given in the book. Progressing from one les ...
Cross-coupling reactions of organoborons with organic halides
... Suzuki-Miyaura reaction is one of the most popular reactions in modern organic chemistry.3 Generally, it is a palladium-catalyzed process of cross-coupling of an organoboron compound with an organic halide which produces a bis-aryl via direct C-C bond formation. The reaction has plenty of applicatio ...
... Suzuki-Miyaura reaction is one of the most popular reactions in modern organic chemistry.3 Generally, it is a palladium-catalyzed process of cross-coupling of an organoboron compound with an organic halide which produces a bis-aryl via direct C-C bond formation. The reaction has plenty of applicatio ...
Document
... Plan: The relationship between Kc and Kp is given by Equation 15.14. To apply that equation, we must determine Δn by comparing the number of moles of product with the number of moles of reactants (Equation 15.15). Solve: There are two moles of gaseous products (2 NH3) and four moles of gaseous react ...
... Plan: The relationship between Kc and Kp is given by Equation 15.14. To apply that equation, we must determine Δn by comparing the number of moles of product with the number of moles of reactants (Equation 15.15). Solve: There are two moles of gaseous products (2 NH3) and four moles of gaseous react ...
PHYSICAL CHEMISTRY IN BRIEF
... the extent of this work is a little broader as our objective was to cover all the major fields of Physical Chemistry. This publication is not intended to substitute for any textbooks or books of examples. Yet we believe that it will prove useful during revision lessons leading up to an exam in Physi ...
... the extent of this work is a little broader as our objective was to cover all the major fields of Physical Chemistry. This publication is not intended to substitute for any textbooks or books of examples. Yet we believe that it will prove useful during revision lessons leading up to an exam in Physi ...
Photochemical and Photophysical Properties of Tetranuclear and
... presence of two luminescence active molecular components in such solutions. Nonetheless, the close analogies between the photophysical properties of the CuqI4L4 clusters in solution and those of crystallographically well-characterizedsolids argues strongly for the integrity of these molecular proper ...
... presence of two luminescence active molecular components in such solutions. Nonetheless, the close analogies between the photophysical properties of the CuqI4L4 clusters in solution and those of crystallographically well-characterizedsolids argues strongly for the integrity of these molecular proper ...
Influence of Temperature on Electrical
... the temperature, but also the ion concentrations for the weak electrolytes (␣ < 1). The effective ion concentrations are calculated according to the law of mass action from the dissociation constant, see Eq. (7). Below, some comments on temperature conversions for several electrolytes which are foun ...
... the temperature, but also the ion concentrations for the weak electrolytes (␣ < 1). The effective ion concentrations are calculated according to the law of mass action from the dissociation constant, see Eq. (7). Below, some comments on temperature conversions for several electrolytes which are foun ...
The effect of the nature of organic acids and the
... The results show that the dissolution of Pb with citric acid alone reached 49% aer 60 min. The addition of sodium citrate aer 20 min of reaction between citric acid and Pb enhanced the dissolution to 62% while sodium citrate alone dissolved 75% of Pb aer the same period of time (Fig. 4a). Sodium ...
... The results show that the dissolution of Pb with citric acid alone reached 49% aer 60 min. The addition of sodium citrate aer 20 min of reaction between citric acid and Pb enhanced the dissolution to 62% while sodium citrate alone dissolved 75% of Pb aer the same period of time (Fig. 4a). Sodium ...
Question Bank for Pre Board Exam(XII Chemistry)
... from a lattice site. 40. Why does conductivity of silicon increase with the rise in temperature? 41.Name the crystal defect which lowers the density of an ionic crystal. 42 What makes the crystal of KCl sometimes appear violet? 43 Which point defect in ionic crystal does not alter the density of the ...
... from a lattice site. 40. Why does conductivity of silicon increase with the rise in temperature? 41.Name the crystal defect which lowers the density of an ionic crystal. 42 What makes the crystal of KCl sometimes appear violet? 43 Which point defect in ionic crystal does not alter the density of the ...
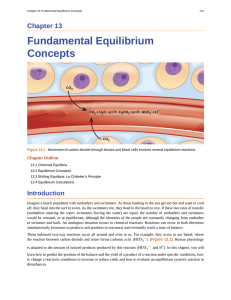
Fundamental Equilibrium Concepts
... • Calculate values of reaction quotients and equilibrium constants, using concentrations and pressures • Relate the magnitude of an equilibrium constant to properties of the chemical system ...
... • Calculate values of reaction quotients and equilibrium constants, using concentrations and pressures • Relate the magnitude of an equilibrium constant to properties of the chemical system ...
Document
... arrangement. When quartz (SiO2 ) is melted and the melt is cooled very rapidly, quartz converted into glass. Que. Why glass is considered as a super cooled liquid? Ans : Amorphous solids have a tendency to flow. Since glass is an amorphous solid , so it is called super cooled liquid or pseudo solid. ...
... arrangement. When quartz (SiO2 ) is melted and the melt is cooled very rapidly, quartz converted into glass. Que. Why glass is considered as a super cooled liquid? Ans : Amorphous solids have a tendency to flow. Since glass is an amorphous solid , so it is called super cooled liquid or pseudo solid. ...
Platinum and Palladium Printing
... processing procedure, since it is also very effective in removing excess unreacted iron(III) from the paper. The description so far has ignored any role played by the cellulose paper substrate. It is likely that the components of the sensitizer will be wholly or partially chemisorbed onto the cellul ...
... processing procedure, since it is also very effective in removing excess unreacted iron(III) from the paper. The description so far has ignored any role played by the cellulose paper substrate. It is likely that the components of the sensitizer will be wholly or partially chemisorbed onto the cellul ...
Grignard knowledge: Alkyl coupling chemistry with inexpensive
... The formation of a Grignard reagent is fascinating to watch. A heap of silvery magnesium turnings swirl in a clattering vortex around the bottom of a glass reaction vessel under a clear and colorless ethereal solvent. The silvery appearance soon changes as the mixture is treated with a clear and col ...
... The formation of a Grignard reagent is fascinating to watch. A heap of silvery magnesium turnings swirl in a clattering vortex around the bottom of a glass reaction vessel under a clear and colorless ethereal solvent. The silvery appearance soon changes as the mixture is treated with a clear and col ...
Unit 8 Chemical Equilibrium Focusing on Acid
... changes, which are balanced because they are occurring at equal rates, within a closed system. What we observe directly is the net effect—neither an increase nor a decrease in any measurable property. Chemistry involves the study of change in chemical substances. To predict and control chemical chan ...
... changes, which are balanced because they are occurring at equal rates, within a closed system. What we observe directly is the net effect—neither an increase nor a decrease in any measurable property. Chemistry involves the study of change in chemical substances. To predict and control chemical chan ...
Preparatory Problems of the 40th IChO - IChO-2016
... Watson kept notes about his adventures with Mr. Sherlock Holmes into the 1950s. An interesting, but incomplete story read as follows: ....and was able to spring into a cab and drive to Baker Street, half afraid that I might be too late to assist at the dénouement of the little mystery. I found Sherl ...
... Watson kept notes about his adventures with Mr. Sherlock Holmes into the 1950s. An interesting, but incomplete story read as follows: ....and was able to spring into a cab and drive to Baker Street, half afraid that I might be too late to assist at the dénouement of the little mystery. I found Sherl ...