Protein structure
... A protein domain is a fundamental unit of organization domains - structural units that fold more or less independently of each other ...
... A protein domain is a fundamental unit of organization domains - structural units that fold more or less independently of each other ...
Biology 211 Intro Molecular and Cell Biology
... removed or added usually requires "sorting signals". These are bits of information carried in the amino acid sequence that sends the proteins to the correct locations in the cell. Example: Signal peptide of hydrophobic amino acids for targeting proteins to endoplasmic reticulum. ...
... removed or added usually requires "sorting signals". These are bits of information carried in the amino acid sequence that sends the proteins to the correct locations in the cell. Example: Signal peptide of hydrophobic amino acids for targeting proteins to endoplasmic reticulum. ...
Section 1.2 Summary – pages 11-18
... • An experimental crop setup is designed to test the hypothesis. • Fertilizer is present in the soil with the experimental plants, but not in the soil with the controls. • All other conditions that are kept the same for both groups- soil, light, water- are constants ...
... • An experimental crop setup is designed to test the hypothesis. • Fertilizer is present in the soil with the experimental plants, but not in the soil with the controls. • All other conditions that are kept the same for both groups- soil, light, water- are constants ...
Press Release, January 11, 2016 Why nerve cells die
... Together with Konstanze Winklhofer and Jörg Tatzelt from the Ruhr-University Bochum, the researchers have expressed artificial aggregation prone proteins as well as Huntington’s diseasecausing mutants of the protein huntingtin in cultured cells. Both types of protein accumulate in large protein depo ...
... Together with Konstanze Winklhofer and Jörg Tatzelt from the Ruhr-University Bochum, the researchers have expressed artificial aggregation prone proteins as well as Huntington’s diseasecausing mutants of the protein huntingtin in cultured cells. Both types of protein accumulate in large protein depo ...
Food Chemistry for 1..
... apart if the temperature is higher • Peptide bonds can also be broken apart by acid ...
... apart if the temperature is higher • Peptide bonds can also be broken apart by acid ...
The Molecular Connection
... The Molecular Connection An Activity in Comparative Biochemistry All living organisms have DNA which codes for sequences of amino acids which form proteins. Proteins that are essential for life such as Cytochrome C (a protein that helps in cellular respiration) take a long time to mutate. This allow ...
... The Molecular Connection An Activity in Comparative Biochemistry All living organisms have DNA which codes for sequences of amino acids which form proteins. Proteins that are essential for life such as Cytochrome C (a protein that helps in cellular respiration) take a long time to mutate. This allow ...
LS50 Section 02 Slides
... What are the building blocks (monomers) of proteins? Monomers: amino acids (20 of these) Which are the functional groups? ...
... What are the building blocks (monomers) of proteins? Monomers: amino acids (20 of these) Which are the functional groups? ...
Organic Molecules
... • collagen and elastin hold body parts together – Movement • actin and myosin allow for muscle contraction ...
... • collagen and elastin hold body parts together – Movement • actin and myosin allow for muscle contraction ...
BIOMOLECULES
... NO other kind of atom can form the number and variety of molecules that ___________________ can because it can bond to 4 other atoms at the same time to make carbohydrates, lipids, nucleic acids, and proteins. A. hydrogen B. oxygen C. carbon D. sodium A ____________________ is made up of a sugar, a ...
... NO other kind of atom can form the number and variety of molecules that ___________________ can because it can bond to 4 other atoms at the same time to make carbohydrates, lipids, nucleic acids, and proteins. A. hydrogen B. oxygen C. carbon D. sodium A ____________________ is made up of a sugar, a ...
gelbank
... patterns. Bio-bag: a “shopping” basket for easy storage of items from the website. FTP site: for access of data in various formats ...
... patterns. Bio-bag: a “shopping” basket for easy storage of items from the website. FTP site: for access of data in various formats ...
doc NUR1 200 Midterm 2006
... near each other in sequence. This relationship is in contrast the three-dimensional conformation, where the amino acid residues involved are: A) B) C) D) E) ...
... near each other in sequence. This relationship is in contrast the three-dimensional conformation, where the amino acid residues involved are: A) B) C) D) E) ...
Ch - Fairview High School
... glucose molecules in same orientation [i.e. CH2OH groups are all on the same side of the chain]. Cellulose= glucose monomers are in _____- configuration. (glycosidic bonds link glucose molecules in alternating upside-down pattern. ...
... glucose molecules in same orientation [i.e. CH2OH groups are all on the same side of the chain]. Cellulose= glucose monomers are in _____- configuration. (glycosidic bonds link glucose molecules in alternating upside-down pattern. ...
The catabolism Carbon Skeleton Amino Acids
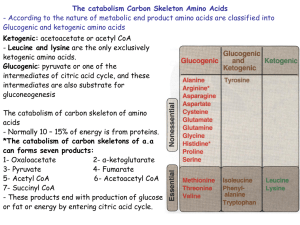
... The catabolism Carbon Skeleton Amino Acids - According to the nature of metabolic end product amino acids are classified into Glucogenic and ketogenic amino acids Ketogenic: acetoacetate or acetyl CoA - Leucine and lysine are the only exclusively ketogenic amino acids. Glucogenic: pyruvate or one of ...
... The catabolism Carbon Skeleton Amino Acids - According to the nature of metabolic end product amino acids are classified into Glucogenic and ketogenic amino acids Ketogenic: acetoacetate or acetyl CoA - Leucine and lysine are the only exclusively ketogenic amino acids. Glucogenic: pyruvate or one of ...
Getting things where they need to go: Protein Targeting
... Highly hydrophobic + charged - charged Hydroxylated Other ...
... Highly hydrophobic + charged - charged Hydroxylated Other ...
Molecules of Life
... bases adenine (A), cytosine (C), guanine (G), and uracil (U); usually single-stranded; functions in protein synthesis and as the genome of some viruses. ...
... bases adenine (A), cytosine (C), guanine (G), and uracil (U); usually single-stranded; functions in protein synthesis and as the genome of some viruses. ...
Protein Synthesis - NCEA Level 2 Biology
... • This makes up part of the structure of the ribosome, the site of protein synthesis in the cytoplasm. • This is the most abundant RNA. • rRNA is made in the nucleolus. • Its function is to hold the mRNA and tRNA together so that a peptide bond can form between the amino acids. ...
... • This makes up part of the structure of the ribosome, the site of protein synthesis in the cytoplasm. • This is the most abundant RNA. • rRNA is made in the nucleolus. • Its function is to hold the mRNA and tRNA together so that a peptide bond can form between the amino acids. ...
Topic 2.1-2.4 Molecular Biology
... substances consisting of covalently bonded amino acids, ready to carry out function – Polypeptides are single AA chains with its own primary structure that may or may not be able to serve a biochemical function without further modification ...
... substances consisting of covalently bonded amino acids, ready to carry out function – Polypeptides are single AA chains with its own primary structure that may or may not be able to serve a biochemical function without further modification ...
Protein Synthesis PPT
... • There are four DNA bases • They code for 20 amino acids • If two bases coded for one amino acid, there wouldn’t be enough, only 16 • Three bases coding for each amino acid is just right, 64 possible combinations. • A set of 3 DNA bases that code for one amino acid is referred to as a codon. ...
... • There are four DNA bases • They code for 20 amino acids • If two bases coded for one amino acid, there wouldn’t be enough, only 16 • Three bases coding for each amino acid is just right, 64 possible combinations. • A set of 3 DNA bases that code for one amino acid is referred to as a codon. ...
Gene Expression - Biology Department | Western Washington
... …the processes by which information contained in genes and genomes is decoded by cells, ...in order to produce molecules that determine the phenotypes observed in organisms, – transcription (post-transcriptional modifications), – translation (post-translational modifications. ...
... …the processes by which information contained in genes and genomes is decoded by cells, ...in order to produce molecules that determine the phenotypes observed in organisms, – transcription (post-transcriptional modifications), – translation (post-translational modifications. ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.