How Much Protein Do You Need
... acids essential versus nonessential it contains. Protein from animal foods is more easily digested than protein form plant foods. A complete protein, which is typically found in animal foods and soy, provides a complete set of the essential amino acids along with some nonessential amino acids. Plant ...
... acids essential versus nonessential it contains. Protein from animal foods is more easily digested than protein form plant foods. A complete protein, which is typically found in animal foods and soy, provides a complete set of the essential amino acids along with some nonessential amino acids. Plant ...
Bio A
... Carbon has lots of properties that make it essential to building lots of different molecules Due to the number of electrons in it’s outer shell, carbon is most likely to make a covalent bond Covalent bonds are formed when an element shares electrons with another molecule. Carbon would need to ...
... Carbon has lots of properties that make it essential to building lots of different molecules Due to the number of electrons in it’s outer shell, carbon is most likely to make a covalent bond Covalent bonds are formed when an element shares electrons with another molecule. Carbon would need to ...
13.2 Ribosomes and Protein Synthesis
... The _______________ of nucleotide ____________ in an mRNA molecule is a set of _____________________ that gives the _____________ in which amino acids should be joined to produce a polypeptide. ...
... The _______________ of nucleotide ____________ in an mRNA molecule is a set of _____________________ that gives the _____________ in which amino acids should be joined to produce a polypeptide. ...
amino acids
... (average size 300 amino acids, Mr 33,000) • Fruit fly (Drosophila melanogaster) about 16,000, humans, other mammals about 40,000 different polypeptides ...
... (average size 300 amino acids, Mr 33,000) • Fruit fly (Drosophila melanogaster) about 16,000, humans, other mammals about 40,000 different polypeptides ...
Necessities of Life Notes
... Name:___________________________________________ Date____________ Pd.: ...
... Name:___________________________________________ Date____________ Pd.: ...
Predicting protein 3D structure from evolutionary sequence variation
... fold classes, ranging in size from 50 to 260 residues., including a G-protein coupled receptor. These blinded inferences are de novo, i.e., they do not use homology modeling or sequence-similar fragments from known structures. The co-evolution signals provide sufficient information to determine accu ...
... fold classes, ranging in size from 50 to 260 residues., including a G-protein coupled receptor. These blinded inferences are de novo, i.e., they do not use homology modeling or sequence-similar fragments from known structures. The co-evolution signals provide sufficient information to determine accu ...
Ch 5 Activity List File
... 9. Identify an ester linkage and describe how it is formed. 10. Distinguish between saturated and unsaturated fats. 11. Name the principal energy storage molecules of plants and animals. 12. Distinguish between a protein and a polypeptide. 13. Explain how a peptide bond forms between two amino acids ...
... 9. Identify an ester linkage and describe how it is formed. 10. Distinguish between saturated and unsaturated fats. 11. Name the principal energy storage molecules of plants and animals. 12. Distinguish between a protein and a polypeptide. 13. Explain how a peptide bond forms between two amino acids ...
Techniques
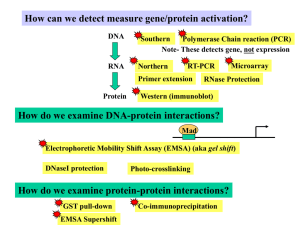
... Techniques to know to understand signal transduction 1. __________- Detect DNA only 2. ___________- Detect RNA 3. ___________- Detect RNA of ____ of expressed genes 4. ________ ( Reverse transcription polymerase chain reaction- to detect RNA) 5. ________________________ Detect protein 6. __________ ...
... Techniques to know to understand signal transduction 1. __________- Detect DNA only 2. ___________- Detect RNA 3. ___________- Detect RNA of ____ of expressed genes 4. ________ ( Reverse transcription polymerase chain reaction- to detect RNA) 5. ________________________ Detect protein 6. __________ ...
Lecture 1 - Temple University
... shared between atoms. The two cases shown represent extremes; often, covalent bonds form with a partial transfer (unequal sharing of electrons), resulting in a polar covalent bond (see Figure 2-43 ...
... shared between atoms. The two cases shown represent extremes; often, covalent bonds form with a partial transfer (unequal sharing of electrons), resulting in a polar covalent bond (see Figure 2-43 ...
Protein Synthesis: Translation
... 3) A transfer RNA with an amino acids is called a charged amino acid. (An enzyme and ATP bind to the correct amino acid to the transfer RNA molecule. At that point it is ready to carry the amino acid to its correct place in the growing polypeptide chain.) ...
... 3) A transfer RNA with an amino acids is called a charged amino acid. (An enzyme and ATP bind to the correct amino acid to the transfer RNA molecule. At that point it is ready to carry the amino acid to its correct place in the growing polypeptide chain.) ...
Proteins
... 4. Quaternary • A. Beta pleated sheet • B. Order of amino acids in a protein • C. A protein with two or more peptide chains • D. The shape of a globular protein • E. Disulfide bonds between R groups ...
... 4. Quaternary • A. Beta pleated sheet • B. Order of amino acids in a protein • C. A protein with two or more peptide chains • D. The shape of a globular protein • E. Disulfide bonds between R groups ...
Introduction to proteomics: analysis of proteins in complex biological
... Proteomics & disease analysis, part I: • Characterize protein differences between disease and normal tissues-– For understanding the disease process; ...
... Proteomics & disease analysis, part I: • Characterize protein differences between disease and normal tissues-– For understanding the disease process; ...
biochemistry/docs/Protein structure 1
... Primary sequence- The amino acid sequence of a polypeptide, listed from N-terminus to C-terminus. Secondary structure- Recurring structural feature of proteins stabilized exclusively by hydrogen bonds between peptide bond elements. Supersecondary structure- Recurring structural feature of proteins c ...
... Primary sequence- The amino acid sequence of a polypeptide, listed from N-terminus to C-terminus. Secondary structure- Recurring structural feature of proteins stabilized exclusively by hydrogen bonds between peptide bond elements. Supersecondary structure- Recurring structural feature of proteins c ...
File
... Lipids are limited to general structural formulas of fats and cell membrane structures and their functions. Proteins are specified to be polymers of amino acids with a variety of functions. These functions are limited to include proteins that relate to structure, such as those found in parts of the ...
... Lipids are limited to general structural formulas of fats and cell membrane structures and their functions. Proteins are specified to be polymers of amino acids with a variety of functions. These functions are limited to include proteins that relate to structure, such as those found in parts of the ...
RIBOSOMES
... -Formed of larger 50S & smaller 30S subunits. 50S :Dome shaped,140-160A in size. -Formed of a central protuberance,a ridge & a stalk. -A valley between central protuberance & ridge. -It has 2 binding sites peptidyl or P& Aminoacyl orA site. 30S:Oval shaped, 90-110A in size. Formed of a platform, hea ...
... -Formed of larger 50S & smaller 30S subunits. 50S :Dome shaped,140-160A in size. -Formed of a central protuberance,a ridge & a stalk. -A valley between central protuberance & ridge. -It has 2 binding sites peptidyl or P& Aminoacyl orA site. 30S:Oval shaped, 90-110A in size. Formed of a platform, hea ...
GoMap
... • Need compiled list of protein acc (all protein databases) and GO terms with evidence -link to BLAST search results • Have GO term assignment linked to InterProScan, in the meantime, link hits to GO via mapping file • Use EC number mappings if your protein hits an enzyme ...
... • Need compiled list of protein acc (all protein databases) and GO terms with evidence -link to BLAST search results • Have GO term assignment linked to InterProScan, in the meantime, link hits to GO via mapping file • Use EC number mappings if your protein hits an enzyme ...
Teacher`s Copy Biochem test prep
... 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. ...
... 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. ...
to find the lecture notes for lecture 4 cellular physiology click here
... 3. Golgi Apparatus = stack of 3 to 20 flattened membrane sacs/cisternae •site of protein modification, and final packaging of the finished protein into secretory vesicles -> exocytosis or for use in the cytosol •movement of protein through the stacks via transport vesicles -definite direction: firs ...
... 3. Golgi Apparatus = stack of 3 to 20 flattened membrane sacs/cisternae •site of protein modification, and final packaging of the finished protein into secretory vesicles -> exocytosis or for use in the cytosol •movement of protein through the stacks via transport vesicles -definite direction: firs ...
Liver funtions part
... 2. New proteins are synthesis for repair work. Like skin’s proteins are synthesized to replace old skin cells are shed away. 3. Some examples of protein names are albumins, globulins, and those essential for blood clotting such as fribrinogen. ...
... 2. New proteins are synthesis for repair work. Like skin’s proteins are synthesized to replace old skin cells are shed away. 3. Some examples of protein names are albumins, globulins, and those essential for blood clotting such as fribrinogen. ...
Prokaryotic Cells, Eukaryotic cells and HIV: Structures, Transcription
... from the ER, modifies them and directs them to other organelles, the plasma membrane or to the exterior of the cell (carried in secretory vesicles). Lysosomes - sites of degradation of macromolecules (a sort of trash can) Peroxisomes - a contained environment for reactions involving hydrogen peroxid ...
... from the ER, modifies them and directs them to other organelles, the plasma membrane or to the exterior of the cell (carried in secretory vesicles). Lysosomes - sites of degradation of macromolecules (a sort of trash can) Peroxisomes - a contained environment for reactions involving hydrogen peroxid ...
Protein modification and trafficking
... Proteins exiting the ER join the Golgi apparatus at the cis Golgi network. The Golgi apparatus consists of a collection of stacked compartments. ...
... Proteins exiting the ER join the Golgi apparatus at the cis Golgi network. The Golgi apparatus consists of a collection of stacked compartments. ...
Working with Data Primary Structure Specifies Tertiary Structure
... After the tertiary structures of proteins were first shown to be highly specific, the question arose as to how the order of amino acids determined the three-dimensional structure. The second protein whose structure was determined was ribonuclease A, an enzyme from cows that was readily available fro ...
... After the tertiary structures of proteins were first shown to be highly specific, the question arose as to how the order of amino acids determined the three-dimensional structure. The second protein whose structure was determined was ribonuclease A, an enzyme from cows that was readily available fro ...
Intracellular Compartments and Protein Sorting
... Signal sequence often at C-terminus Some proteins with sequence near N-terminus Peroxins (receptors, docking proteins) participate in transport Inherited defects in peroxin genes such as Zellweger syndrome ...
... Signal sequence often at C-terminus Some proteins with sequence near N-terminus Peroxins (receptors, docking proteins) participate in transport Inherited defects in peroxin genes such as Zellweger syndrome ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.