2.3 Study Guide - Issaquah Connect
... broken down as a source of chemical energy; part of cell structure functions including movement, transport, chemical catalysts ...
... broken down as a source of chemical energy; part of cell structure functions including movement, transport, chemical catalysts ...
2.4 Molecules to Metabolism NOTES - Proteins
... polypeptides. • There are 20 different amino acids in polypeptides synthesized on ribosomes. • Amino acids can be linked together in any sequence giving a huge range of possible polypeptides. Most organisms use the same 20 amino acids in the same genetic code although there are some exceptions. Spec ...
... polypeptides. • There are 20 different amino acids in polypeptides synthesized on ribosomes. • Amino acids can be linked together in any sequence giving a huge range of possible polypeptides. Most organisms use the same 20 amino acids in the same genetic code although there are some exceptions. Spec ...
Prokaryotic vs eukaryotic: prokaryotic – no internal
... organelles (they only have ribosomes) and no nucleus; their chromosomes are circular and do not have histone proteins; bacteria and archeae are the only examples. Eukaryotic – have organelles; DNA in linear chromosomes within a nucleus; Key organelles to know functions of: mitochondria, chloroplasts ...
... organelles (they only have ribosomes) and no nucleus; their chromosomes are circular and do not have histone proteins; bacteria and archeae are the only examples. Eukaryotic – have organelles; DNA in linear chromosomes within a nucleus; Key organelles to know functions of: mitochondria, chloroplasts ...
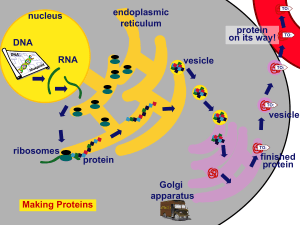
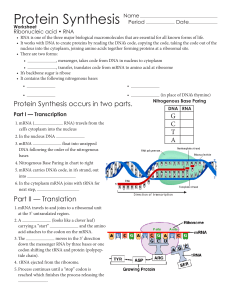
Protein Synthesis
... The ribosome is made of protein and ribosomal RNA (rRNA). All cells need proteins, DNA, and ribosomes. Prokaryotes & Eukaryotes have ribosomes. ...
... The ribosome is made of protein and ribosomal RNA (rRNA). All cells need proteins, DNA, and ribosomes. Prokaryotes & Eukaryotes have ribosomes. ...
last year`s final exam
... 18) What microtubule motor helps move secretory vesicles toward the cell membrane? 19) What happens in the E site of ribosomes? 20) Where does phosphatidylserine get synthesized? 21) What happens if phosphatidylserine is on the outside of a cell? 22) What is meant by, “The ribosome is a ribozyme”? 2 ...
... 18) What microtubule motor helps move secretory vesicles toward the cell membrane? 19) What happens in the E site of ribosomes? 20) Where does phosphatidylserine get synthesized? 21) What happens if phosphatidylserine is on the outside of a cell? 22) What is meant by, “The ribosome is a ribozyme”? 2 ...
3D modelling activity
... proteins potential tertiary structures with software. This requires a great deal of computing power to accurately model and must be done using large servers with massive data bases. You can do this through the submission of a sequence (DNA/AA) as a fasta file to services such as PHYRE2, which will g ...
... proteins potential tertiary structures with software. This requires a great deal of computing power to accurately model and must be done using large servers with massive data bases. You can do this through the submission of a sequence (DNA/AA) as a fasta file to services such as PHYRE2, which will g ...
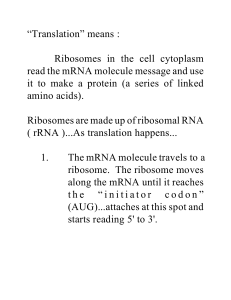
“Translation” means : Ribosomes in the cell cytoplasm read the
... 3. As the ribosome moves along mRNA, codons of bases are read, each one is matched up with the right tRNA and amino acid combo. As the ribosome keeps reading, and tRNA’s are being lined up, a string of amino acids are lined up. They are joined with “peptide bonds” and voila ! You have a protein. ...
... 3. As the ribosome moves along mRNA, codons of bases are read, each one is matched up with the right tRNA and amino acid combo. As the ribosome keeps reading, and tRNA’s are being lined up, a string of amino acids are lined up. They are joined with “peptide bonds” and voila ! You have a protein. ...
Title - Iowa State University
... D. Sugars 8.) An Enzyme speeds up an reaction by ___________ A. decrease activation energy of a reaction. B. increase the ΔG of a reaction C. decrease the Δ G of a reaction D. increase the activation energy of the reaction 9.) Polypeptide chains are always synthesized from _______ to _______. a.) hy ...
... D. Sugars 8.) An Enzyme speeds up an reaction by ___________ A. decrease activation energy of a reaction. B. increase the ΔG of a reaction C. decrease the Δ G of a reaction D. increase the activation energy of the reaction 9.) Polypeptide chains are always synthesized from _______ to _______. a.) hy ...
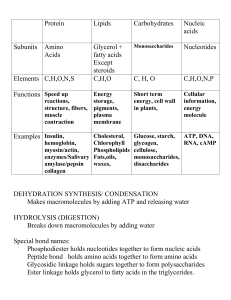
PROTEINS:
... In the solid state amino acids exist entirely in the dipolar form callled zwitterion. Amino acids are internally neutralized molecule. Since amino acids has its own proton donating group, NH3+and has its own proton accepting group, COO- so these dipolar ions can neutralize acids or bases of sufficie ...
... In the solid state amino acids exist entirely in the dipolar form callled zwitterion. Amino acids are internally neutralized molecule. Since amino acids has its own proton donating group, NH3+and has its own proton accepting group, COO- so these dipolar ions can neutralize acids or bases of sufficie ...
PowerPoint
... polysaccharides (starch, glycogen, and cellulose), and lipid (?, with different synthesizing method) •Macromolecules are responsible for most of the form and function in living ystems. They are, however, generated by polymerization of small organic molecules, a fundamental principle of cellular chem ...
... polysaccharides (starch, glycogen, and cellulose), and lipid (?, with different synthesizing method) •Macromolecules are responsible for most of the form and function in living ystems. They are, however, generated by polymerization of small organic molecules, a fundamental principle of cellular chem ...
Slide 1
... •site of ATP synthesis •energy of ATP fuels most of the chemical reactions in a cell •surrounded by a double membrane; outer membrane serves as a boundary and inner membrane is highly folded to form cristae, which increase the surface area available for chemical reactions that occur in the mito ...
... •site of ATP synthesis •energy of ATP fuels most of the chemical reactions in a cell •surrounded by a double membrane; outer membrane serves as a boundary and inner membrane is highly folded to form cristae, which increase the surface area available for chemical reactions that occur in the mito ...
Lecture 13-Effects of glycosylation on protein structure and function
... • Treatment of CD2 with PNGase to remove all N-‐ linked carbohydrate results in complete loss of binding to CD58 • Muta3on of the glycosyla3on site in the N-‐ terminal domain, either by changing the g ...
... • Treatment of CD2 with PNGase to remove all N-‐ linked carbohydrate results in complete loss of binding to CD58 • Muta3on of the glycosyla3on site in the N-‐ terminal domain, either by changing the g ...
Teacher practical Make your own protein Specification references
... a The mutation can change an amino acid in the protein chain. This can affect the bending and folding of the protein, changing its shape. b The function of the protein depends on its shape, for example, the active site shape in an enzyme. If you change the shape, you change the function. Some mutati ...
... a The mutation can change an amino acid in the protein chain. This can affect the bending and folding of the protein, changing its shape. b The function of the protein depends on its shape, for example, the active site shape in an enzyme. If you change the shape, you change the function. Some mutati ...
Macromolecule Notes Powerpoint
... • Cellulose and chitin are used in plants and animals for constructing cell walls and exoskeletons. We don’t have the enzymes that recognize how the glucose molecules are hooked together in this form so we don’t ...
... • Cellulose and chitin are used in plants and animals for constructing cell walls and exoskeletons. We don’t have the enzymes that recognize how the glucose molecules are hooked together in this form so we don’t ...
Lecture 9
... Polypeptide subunits associate in a geometrically specific manner. Why subunits? Easier to repair self-assembling single subunit vs. a large polypeptide. • Increasing a protein’s size through subunits is more efficient for specifying the active site. • Provides a structural basis for regulating acti ...
... Polypeptide subunits associate in a geometrically specific manner. Why subunits? Easier to repair self-assembling single subunit vs. a large polypeptide. • Increasing a protein’s size through subunits is more efficient for specifying the active site. • Provides a structural basis for regulating acti ...
Food Utilization
... • Carbohydrates and proteins, about 4 kcal/g – sugar and alcohol are “empty” calories Activity ...
... • Carbohydrates and proteins, about 4 kcal/g – sugar and alcohol are “empty” calories Activity ...
Macromolecule Notes - Ms. Dooley`s Science Class
... • Cellulose and chitin are used in plants and animals for constructing cell walls and exoskeletons. We don’t have the enzymes that recognize how the glucose molecules are hooked together in this form so we don’t ...
... • Cellulose and chitin are used in plants and animals for constructing cell walls and exoskeletons. We don’t have the enzymes that recognize how the glucose molecules are hooked together in this form so we don’t ...
Transcription and Translation computer lab test review
... During transcription, RNA is developed from a strand of DNA. List the base pairs used to make RNA. What is the name of the DNA strand used in transcription? Where does transcription occur? Where does translation occur? Name the RNA codon that is used to start translation. Which three codons will sto ...
... During transcription, RNA is developed from a strand of DNA. List the base pairs used to make RNA. What is the name of the DNA strand used in transcription? Where does transcription occur? Where does translation occur? Name the RNA codon that is used to start translation. Which three codons will sto ...
Word of the Day
... RNA polymerase unzips DNA and copies it into RNA. A’s connect with U’s and G’s connect with C’s. The starting point of transcription is known as the Promoter, the end is known as the terminal signal. After transcription the mRNA moves into the cytosol for protein synthesis. ...
... RNA polymerase unzips DNA and copies it into RNA. A’s connect with U’s and G’s connect with C’s. The starting point of transcription is known as the Promoter, the end is known as the terminal signal. After transcription the mRNA moves into the cytosol for protein synthesis. ...
Organic Molecules
... that an organism does. These functions include structural support, storage, transport of other substances, intercellular signaling, movement, and defense against foreign substances. ...
... that an organism does. These functions include structural support, storage, transport of other substances, intercellular signaling, movement, and defense against foreign substances. ...
Homology
... paralogs and their distribution in genomes provides clues on the way genomes evolved. Gen and genome duplication have emerged as the most important pathway to molecular innovation, including the evolution of developmental pathways. Xenologs: gene was obtained by organism through horizontal transfer. ...
... paralogs and their distribution in genomes provides clues on the way genomes evolved. Gen and genome duplication have emerged as the most important pathway to molecular innovation, including the evolution of developmental pathways. Xenologs: gene was obtained by organism through horizontal transfer. ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.