Organometallic compounds of the d-block elements
... Odd numbered -systems have a non-bonding orbital in the middle of the energy range with a node in the middle. ...
... Odd numbered -systems have a non-bonding orbital in the middle of the energy range with a node in the middle. ...
Organometallics - X-Ray - University of Kentucky
... allows for the isolation of (PNP)TaMe4 (3). (PNP)TaMe4 (3) eVolVes thermally and/or photochemically into a bis(methylidene) complex (PNP)Ta(dCH2)2 (4). The identity of the latter has been established by X-ray structural, NMR spectroscopic, and DFT computational studies. It does not appear that 4 pos ...
... allows for the isolation of (PNP)TaMe4 (3). (PNP)TaMe4 (3) eVolVes thermally and/or photochemically into a bis(methylidene) complex (PNP)Ta(dCH2)2 (4). The identity of the latter has been established by X-ray structural, NMR spectroscopic, and DFT computational studies. It does not appear that 4 pos ...
NAME: Chem 1b, 2005, 3rd
... A periodic table and useful data are provided on the insert. You may use the insert for scratch work but enter all work to be graded in the space with each question. 1) (58 points) For each of the following species, give its IUPAC chemical name and the hybridization of the transition-metal atom. Dra ...
... A periodic table and useful data are provided on the insert. You may use the insert for scratch work but enter all work to be graded in the space with each question. 1) (58 points) For each of the following species, give its IUPAC chemical name and the hybridization of the transition-metal atom. Dra ...
Descriptive Chemistry of Elements d-Block
... The filling of electrons into the d-levels creates the d-block. In a free metal, the nd-electrons fill after the filling of (n+1)s-electrons and before the filling of (n+1)p-electrons, thus, the d-block elements are situated between those of s- and p-block elements. Generally, the electron configura ...
... The filling of electrons into the d-levels creates the d-block. In a free metal, the nd-electrons fill after the filling of (n+1)s-electrons and before the filling of (n+1)p-electrons, thus, the d-block elements are situated between those of s- and p-block elements. Generally, the electron configura ...
transition metal complexes
... 1.! More than one oxidation state 2.! Many compounds are colored 3.! Interesting magnetic properties 4.! Form Metal complexes or Coordination compounds 5.! Transition metals play important roles in biological systems and modern technology. ...
... 1.! More than one oxidation state 2.! Many compounds are colored 3.! Interesting magnetic properties 4.! Form Metal complexes or Coordination compounds 5.! Transition metals play important roles in biological systems and modern technology. ...
What Should Be Impossible: Resolution of the Mononuclear Gallium
... “labile” metal ion. We report here the resolution of simple complexes of this cation. The hydroxamate functional group (R(OH)NC(O)R′) is a common iron-binding motif found in siderophores, small molecules synthesized by bacteria as iron-transport agents. Deprotonation of the N(OH) to give the hydroxa ...
... “labile” metal ion. We report here the resolution of simple complexes of this cation. The hydroxamate functional group (R(OH)NC(O)R′) is a common iron-binding motif found in siderophores, small molecules synthesized by bacteria as iron-transport agents. Deprotonation of the N(OH) to give the hydroxa ...
슬라이드 1
... The more easily reduced, the more reactive is the compound toward cuprate reagents. Compounds such as a,b-unsaturated esters and nitriles, which are not as easily reduced as the corresponding ketones, do not react as readily with dialkyl cuprates, even though they are good Michael acceptors in class ...
... The more easily reduced, the more reactive is the compound toward cuprate reagents. Compounds such as a,b-unsaturated esters and nitriles, which are not as easily reduced as the corresponding ketones, do not react as readily with dialkyl cuprates, even though they are good Michael acceptors in class ...
Chem+174–Lecture4c
... of triphenylphosphine yields the cis isomer, which can be considered as the kinetic product The cis product is converted into the trans isomer at elevated temperature, which makes it the thermodynamic product The piperidine adduct can be used as reactant with other phosphine and phosphonite liga ...
... of triphenylphosphine yields the cis isomer, which can be considered as the kinetic product The cis product is converted into the trans isomer at elevated temperature, which makes it the thermodynamic product The piperidine adduct can be used as reactant with other phosphine and phosphonite liga ...
Chapter 25 The Chemistry of Life: Organic Chemistry 25.1 Some
... Even though they contain π bonds, aromatic hydrocarbons undergo substitution reactions more readily than addition reactions. ...
... Even though they contain π bonds, aromatic hydrocarbons undergo substitution reactions more readily than addition reactions. ...
(i) Coordination entity
... STEREOISOMERISM: Stereo isomers have the same chemical formula and chemical bonds but they have different spatial arrangement. They are of two kinds A. Geometrical isomerism B. Optical isomerism GEOMETRICAL ISOMERISM- This type of isomerism arises in heteroleptic complexes due to different possible ...
... STEREOISOMERISM: Stereo isomers have the same chemical formula and chemical bonds but they have different spatial arrangement. They are of two kinds A. Geometrical isomerism B. Optical isomerism GEOMETRICAL ISOMERISM- This type of isomerism arises in heteroleptic complexes due to different possible ...
Topic 13.2 Periodicity First Row d
... chemical reactions. The transition metals form complex ions with ligands that can donate lone pairs of electrons. This results in close contact between the metal ion and the ligand. Heterogeneous (different state) catalysts are more common than homogeneous. ...
... chemical reactions. The transition metals form complex ions with ligands that can donate lone pairs of electrons. This results in close contact between the metal ion and the ligand. Heterogeneous (different state) catalysts are more common than homogeneous. ...
coordination compounds
... • As is the case with ionic compounds, the name of the cation appears first; the anion is named last. • Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing. ...
... • As is the case with ionic compounds, the name of the cation appears first; the anion is named last. • Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing. ...
Syntheses of Variations of Stereogenic-at
... test to determine whether a given catalyst can produce a polymer with a single structure. Among the tested catalysts 6c led to highly structured (95 %) cis, syndio poly(DCMNBD) as expected. Polymerization of DCMNBD with 6a,b,d, 7a,b, and 8, resulted in less structured species. On the other hand, the ...
... test to determine whether a given catalyst can produce a polymer with a single structure. Among the tested catalysts 6c led to highly structured (95 %) cis, syndio poly(DCMNBD) as expected. Polymerization of DCMNBD with 6a,b,d, 7a,b, and 8, resulted in less structured species. On the other hand, the ...
Building Molecular Orbitals for a Square Pyramidal Oxorhenium(V
... 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s group in Organometallics 2015, 34, 3152-3158. This complex is reported to have a “distorted” square pyramidal geometry. For the purpose of this exercise, first consider the structure to be an “ideal” square pyramid of formula M(L)3 ...
... 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s group in Organometallics 2015, 34, 3152-3158. This complex is reported to have a “distorted” square pyramidal geometry. For the purpose of this exercise, first consider the structure to be an “ideal” square pyramid of formula M(L)3 ...
Activity - IONiC / VIPEr
... a. How are the orbitals of metal d character affected in your MO diagram by the absence of a ligand L on the z axis? b. Redraw your MO diagram for orbitals of metal d character in a square pyramidal complex. Label the orbitals. 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s gro ...
... a. How are the orbitals of metal d character affected in your MO diagram by the absence of a ligand L on the z axis? b. Redraw your MO diagram for orbitals of metal d character in a square pyramidal complex. Label the orbitals. 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison’s gro ...
synthesis, characterization and applications of metal complexes of 5
... The synthesized complexes of Nitro-SAMT were screened for their invitro antibacterial activity against pathogenic strains of gram negative bacteria such as E.Coli, Pseudomonas aeruginosa, salmonella typhi and shigella flexneri using plate technique. The bacterial were cultured (15 mm dia) in previou ...
... The synthesized complexes of Nitro-SAMT were screened for their invitro antibacterial activity against pathogenic strains of gram negative bacteria such as E.Coli, Pseudomonas aeruginosa, salmonella typhi and shigella flexneri using plate technique. The bacterial were cultured (15 mm dia) in previou ...
PP Aldehyde and ketone
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
Aldehydes and Ketones
... Strong bases (KH or NaNH2) will cause complete ionization to enolate. O ...
... Strong bases (KH or NaNH2) will cause complete ionization to enolate. O ...
IOSR Journal of Applied Chemistry (IOSRJAC)
... wherein nitrogen of NO takes away alongwith its lone pair of electrons as well as radical electron from the nitrogen of the ring (Step-II) and cation (II) is formed. This is in consonance with the fact that nitrosyl ligand is formally regarded sometimes as NO+, sometimes as NO-, but is usually elimi ...
... wherein nitrogen of NO takes away alongwith its lone pair of electrons as well as radical electron from the nitrogen of the ring (Step-II) and cation (II) is formed. This is in consonance with the fact that nitrosyl ligand is formally regarded sometimes as NO+, sometimes as NO-, but is usually elimi ...
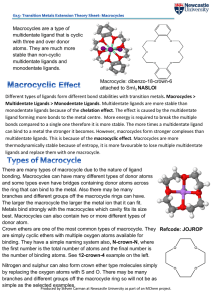
Different types of ligands form different bond stabilities with transition
... bonding. Macrocycles can have many different types of donor atoms and some types even have bridges containing donor atoms across the ring that can bind to the metal. Also there may be many branches and different groups off the macrocycle rings can have. The larger the macrocycle the larger the metal ...
... bonding. Macrocycles can have many different types of donor atoms and some types even have bridges containing donor atoms across the ring that can bind to the metal. Also there may be many branches and different groups off the macrocycle rings can have. The larger the macrocycle the larger the metal ...
Lecture6-Organometallic Chemistry
... are coordinatively unsaturated (having an open coordination site or being weakly coordinated) Square-planar 16-electron complexes are coordinatively unsaturated ML4 complexes of Pd(II), Pt(II) and Rh(I) [RhCl(PPh3)3] – hydrogenation catalyst ...
... are coordinatively unsaturated (having an open coordination site or being weakly coordinated) Square-planar 16-electron complexes are coordinatively unsaturated ML4 complexes of Pd(II), Pt(II) and Rh(I) [RhCl(PPh3)3] – hydrogenation catalyst ...
17.The d-Block Elements.General properties
... oxidation states due to tendency of (n-1)d as well as ns electrons to take part in bond formation. • Magnetic properties: Most of transition metals are paramagnetic in nature due to presence of unpaired electrons. It increase s from Sc to Cr and then decreases because number of unpaired and then dec ...
... oxidation states due to tendency of (n-1)d as well as ns electrons to take part in bond formation. • Magnetic properties: Most of transition metals are paramagnetic in nature due to presence of unpaired electrons. It increase s from Sc to Cr and then decreases because number of unpaired and then dec ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.