A Chapter 3
... Common in square planar and octahedral complexes but not in tetrahedral complexes because all tetrahedral complexes [such as Ma4 , Ma2b2 , Mabcd, where a,b,c,d represents ligands ]exist in only one geometric form in which all positions are adjacent to each other. Existence of two different compounds ...
... Common in square planar and octahedral complexes but not in tetrahedral complexes because all tetrahedral complexes [such as Ma4 , Ma2b2 , Mabcd, where a,b,c,d represents ligands ]exist in only one geometric form in which all positions are adjacent to each other. Existence of two different compounds ...
PPT file
... electrophile is added (leaving neutral acetone molecules as the only available electrophiles) O ...
... electrophile is added (leaving neutral acetone molecules as the only available electrophiles) O ...
Schedule • Lecture 1: Electronic absorption spectroscopy • Lecture 2
... ¾ metal is easily reduced (for example metal in high oxidation state) ¾ ligand is easily oxidized If they occur in the visible or near-ultraviolet, they are much more intense than ‘d-d’ bands and the latter will not be seen ...
... ¾ metal is easily reduced (for example metal in high oxidation state) ¾ ligand is easily oxidized If they occur in the visible or near-ultraviolet, they are much more intense than ‘d-d’ bands and the latter will not be seen ...
Synthesis and Structural Aspects of Copper (II), Cobalt (II), and
... The IR data of these complexes show: (i) a very intense and broad O-H stretching band at 3380 cm-1, (ii) two C-H stretching bands at 2930-2985 cm-1, (iii) a C=O stretching band at 1680 cm-1 indicative of the presence of a free carboxylate anion, (iv) the lack of bands in the region 1750-1700 cm-1 (i ...
... The IR data of these complexes show: (i) a very intense and broad O-H stretching band at 3380 cm-1, (ii) two C-H stretching bands at 2930-2985 cm-1, (iii) a C=O stretching band at 1680 cm-1 indicative of the presence of a free carboxylate anion, (iv) the lack of bands in the region 1750-1700 cm-1 (i ...
The Other Side of NHCs: NHCs in Transition Metal Catalysis
... •When forming air sensitive metal complexes, protecting the free carbene with an alcohol or chloroform proves useful. •Not all azolium salts can be protected in this manner—unsaturated NHCs are deprotonated. •Coordination to metal may involve carbene dimer and Wanzlick equilibrium. X R ...
... •When forming air sensitive metal complexes, protecting the free carbene with an alcohol or chloroform proves useful. •Not all azolium salts can be protected in this manner—unsaturated NHCs are deprotonated. •Coordination to metal may involve carbene dimer and Wanzlick equilibrium. X R ...
Why Cyanide Pretends To Be A Weak
... qualitatively reproduce the experimentally observed properties of this system quite well. We have also opened “Pandora’s Box” when it comes to truly understanding how the different factors affecting spin-state equilibrium come together, as exemplified in the Cr(II)/Fe(II) discussion above. Additiona ...
... qualitatively reproduce the experimentally observed properties of this system quite well. We have also opened “Pandora’s Box” when it comes to truly understanding how the different factors affecting spin-state equilibrium come together, as exemplified in the Cr(II)/Fe(II) discussion above. Additiona ...
Chromium Arene Complexes
... Under thermodynamic control, the equilibrium depends on steric and electronic interactions in the intermediate anions. Donor/acceptors are usually attacked at a meta-position Bulky groups direct to para. Coordinating groups direct to ortho. ...
... Under thermodynamic control, the equilibrium depends on steric and electronic interactions in the intermediate anions. Donor/acceptors are usually attacked at a meta-position Bulky groups direct to para. Coordinating groups direct to ortho. ...
Synthesis and characterization of transition metal coordination
... The main IR frequencies can be seen in Table 2. The IR spectra of the prepared compounds show 2 bands in the ranges of 1552-1572 and 1374-1380 cm −1 , characteristic of the asymmetric and symmetric stretching vibrations of the carboxylic groups of BDC coordinated to the metal center. The separation ...
... The main IR frequencies can be seen in Table 2. The IR spectra of the prepared compounds show 2 bands in the ranges of 1552-1572 and 1374-1380 cm −1 , characteristic of the asymmetric and symmetric stretching vibrations of the carboxylic groups of BDC coordinated to the metal center. The separation ...
FTIR Spectoscopy of Ca++ DOPED Alq3 OLED Phospor
... 3.2. FTIR spectroscopy of Ca doped Alq3 vibrations, whereas below 648.10 cm–1 the central part FT-IR is an analytical technique which confirms the around the Al-atom becomes more important. In the range of molecular structures of the synthesized complexes. This 4000-2000, the broad peak at 3401.09 r ...
... 3.2. FTIR spectroscopy of Ca doped Alq3 vibrations, whereas below 648.10 cm–1 the central part FT-IR is an analytical technique which confirms the around the Al-atom becomes more important. In the range of molecular structures of the synthesized complexes. This 4000-2000, the broad peak at 3401.09 r ...
J. Am. Chem. Soc. 2000, 122, 10714
... Py, the melting temperatures of duplexes containing mismatches with the natural bases were measured. The data in Table 1 show that the duplex containing the Dipic:Py base pair is considerably more stable than those containing mismatches with one of the natural bases. Relative to Dipic:Py, the mispai ...
... Py, the melting temperatures of duplexes containing mismatches with the natural bases were measured. The data in Table 1 show that the duplex containing the Dipic:Py base pair is considerably more stable than those containing mismatches with one of the natural bases. Relative to Dipic:Py, the mispai ...
Summary of Thesis INFLUENCE OF POLAR SOLVENTS UPON THE
... values for the formed complexes are determined. Since chloroform is used as solvent in extraction experiments, the complex formation of alkali metal cations and ammonium as dibenzyldithiocarbamate salts with crown ethers in chloroform is systematically investigated by means of calorimetric titration ...
... values for the formed complexes are determined. Since chloroform is used as solvent in extraction experiments, the complex formation of alkali metal cations and ammonium as dibenzyldithiocarbamate salts with crown ethers in chloroform is systematically investigated by means of calorimetric titration ...
ppt
... with triphenylphosphine (Ph3P:) to give a phosphonium salt. The protons on the carbon adjacent to phosphorous are acidic. Ph3P ...
... with triphenylphosphine (Ph3P:) to give a phosphonium salt. The protons on the carbon adjacent to phosphorous are acidic. Ph3P ...
RuP(OMe) 2
... Hemilabile ligands have been of great interest to chemists working toward the development of molecular sensors. Hemilabile coordination is found to occur amongst polydentate ligands that contain both chemically inert and labile sites bound to a metal center. In the presence of molecules with a stron ...
... Hemilabile ligands have been of great interest to chemists working toward the development of molecular sensors. Hemilabile coordination is found to occur amongst polydentate ligands that contain both chemically inert and labile sites bound to a metal center. In the presence of molecules with a stron ...
review sheet plus practice problems
... Questions that may appear on the exam: What is the name for this alkyl halide / alcohol / ether? Is an alcohol 1°, 2°, or 3°? What are the products of free radical halogenation of an alkane (ex: Cl2/uv light)? Give the chain mechanism for free radical halogenation. What is the selectivity for bromin ...
... Questions that may appear on the exam: What is the name for this alkyl halide / alcohol / ether? Is an alcohol 1°, 2°, or 3°? What are the products of free radical halogenation of an alkane (ex: Cl2/uv light)? Give the chain mechanism for free radical halogenation. What is the selectivity for bromin ...
Coordination Chemistry
... [Co(NH3)6]Cl3 [Co(NH3)5Cl]Cl2 [Co(NH3)4Cl2]Cl [Co(NH3)4Cl2]Cl primary valence (formal oxidation state) ...
... [Co(NH3)6]Cl3 [Co(NH3)5Cl]Cl2 [Co(NH3)4Cl2]Cl [Co(NH3)4Cl2]Cl primary valence (formal oxidation state) ...
Hard and Soft Acids and Bases
... What is obvious here is that the soft Ag+ ion prefers the soft SH¯ ligand to the hard OH¯ ligand, whereas for the hard Ga3+ ion the opposite is true. The intermediate Pb2+ ion has no strong preference. Another set of examples is given by: Metal ion: ...
... What is obvious here is that the soft Ag+ ion prefers the soft SH¯ ligand to the hard OH¯ ligand, whereas for the hard Ga3+ ion the opposite is true. The intermediate Pb2+ ion has no strong preference. Another set of examples is given by: Metal ion: ...
Observation of back-donation in 3d metal cyanide complexes
... ligands at similar energies. This experimental fact indicates obviously that the CN 2 anion is weakly affected by the 3d atom–ligand bonding. Thus, it can be considered as a stable fragment (a quasimolecule) interacting with the central 3d atom. In the vicinity of the p * 2p resonance (additional ba ...
... ligands at similar energies. This experimental fact indicates obviously that the CN 2 anion is weakly affected by the 3d atom–ligand bonding. Thus, it can be considered as a stable fragment (a quasimolecule) interacting with the central 3d atom. In the vicinity of the p * 2p resonance (additional ba ...
Potintiometric and Thermodynamic Studies of Some Divalent Metal
... the hypothesis that a large number of water molecules are released upon complexation with the probability of a change of the coordination number28. This was supported by the values of H, where it was found that -H1>-H2 for the complexes, except for Zn(II) and Cd(II)-complexes. The lower negative ...
... the hypothesis that a large number of water molecules are released upon complexation with the probability of a change of the coordination number28. This was supported by the values of H, where it was found that -H1>-H2 for the complexes, except for Zn(II) and Cd(II)-complexes. The lower negative ...
Overview of the Reactions of Carbonyl Compounds
... • H- attacks the carbonyl carbon. The alkoxide anion is then protonated by dilute acid. • Hydride additions are irreversible because a hydride is not a good leaving group • LiAlH4 and NaBH4 react as donors of hydride ion (H-) ...
... • H- attacks the carbonyl carbon. The alkoxide anion is then protonated by dilute acid. • Hydride additions are irreversible because a hydride is not a good leaving group • LiAlH4 and NaBH4 react as donors of hydride ion (H-) ...
Carbonyls - wellswaysciences
... Oxidation is: Gain of oxygen Loss of hydrogen Loss of electrons In the oxidation of alcohols or aldehydes, ion electron equations can be written which are more informative than the [O] symbol. • See p. 23 in A2 text. ...
... Oxidation is: Gain of oxygen Loss of hydrogen Loss of electrons In the oxidation of alcohols or aldehydes, ion electron equations can be written which are more informative than the [O] symbol. • See p. 23 in A2 text. ...
Chapter 17: Aldehydes and Ketones: Nucleophilic Addition to the
... with triphenylphosphine (Ph3P:) to give a phosphonium salt.The protons on the carbon adjacent to phosphorous are acidic. Ph3P ...
... with triphenylphosphine (Ph3P:) to give a phosphonium salt.The protons on the carbon adjacent to phosphorous are acidic. Ph3P ...
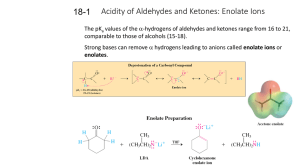
18-1 Enolates (PPT)
... For ordinary aldehydes and ketones, only traces of the enol form are present. The enol form is less stable by 8-12 kcal mol-1. However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more subs ...
... For ordinary aldehydes and ketones, only traces of the enol form are present. The enol form is less stable by 8-12 kcal mol-1. However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more subs ...
14. The complexometric determination of calcium and magnesium in
... titration. Complexes (also called "coordination compounds" or "metal complexes") are structures consisting of a central atom or molecule connected to surrounding atoms or molecules (ligands) by coordination bonds. Most often used reagent in complexometric analyses is EDTA - EthyleneDiamineTetraAceti ...
... titration. Complexes (also called "coordination compounds" or "metal complexes") are structures consisting of a central atom or molecule connected to surrounding atoms or molecules (ligands) by coordination bonds. Most often used reagent in complexometric analyses is EDTA - EthyleneDiamineTetraAceti ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.