Carbenes and Nitrenes: Structure, generaNon and reacNvity
... early as 1943 (J. Pharm. Soc. Jpn. 1943, 63, 296) and metal complexes of NHCs were already reported in the late 60’s. However, it is only in the last two decades (milestone: 1991, Arduengo, first X-‐ra ...
... early as 1943 (J. Pharm. Soc. Jpn. 1943, 63, 296) and metal complexes of NHCs were already reported in the late 60’s. However, it is only in the last two decades (milestone: 1991, Arduengo, first X-‐ra ...
MOLECULAR NITRIDES CONTAINING TITANIUM AND GROUP 1, 2
... Treatment of the metalloligand 1 with mercury diiodide in toluene at room temperature leads to the adduct [I2Hg{(µ3-NH)3Ti3Cp*3(µ3-N)}] (52). The reaction of complex 52 with [K{N(SiMe3)2}] gives the compound [Hg2{(µ3-N)2(µNH)Ti3Cp*3(µ3-N)}2] (53), which shows two mercury atoms bridging two incomplet ...
... Treatment of the metalloligand 1 with mercury diiodide in toluene at room temperature leads to the adduct [I2Hg{(µ3-NH)3Ti3Cp*3(µ3-N)}] (52). The reaction of complex 52 with [K{N(SiMe3)2}] gives the compound [Hg2{(µ3-N)2(µNH)Ti3Cp*3(µ3-N)}2] (53), which shows two mercury atoms bridging two incomplet ...
Co-ordination Chemistry with Macrocyclic Compounds
... The association of two or more species by noncovalent bonds constituted what has been designated by supramolecular chemistry or host guest chemistry, and extends the Fischer's "lock and key" concept from steric fit to other molecular properties [15,20, 21]. Among macrocycles there is a vast number o ...
... The association of two or more species by noncovalent bonds constituted what has been designated by supramolecular chemistry or host guest chemistry, and extends the Fischer's "lock and key" concept from steric fit to other molecular properties [15,20, 21]. Among macrocycles there is a vast number o ...
`Relaxing` The Orbital Selection Rule
... depends on Doct and B for ligand-field spectra decides colour of molecule The width of a band relates to the vibrational excitation that accompanies the electronic transition: narrow bands: excited state has similar geometry to the ground state broad bands: excited state has different geomet ...
... depends on Doct and B for ligand-field spectra decides colour of molecule The width of a band relates to the vibrational excitation that accompanies the electronic transition: narrow bands: excited state has similar geometry to the ground state broad bands: excited state has different geomet ...
Synthesis and physico-chemical studies of newly formed
... Few complexes of Transition and Inner transition metals have been synthesized by reacting their metal salts with (5-methoxy- 2- {{(4-Methoxy-3, 5-dimethy- 1-pyridiny) methyl} sulfinyI}-IHbenzimidazole). All the complexes were synthesized in ethanolic medium and refluxed in reaction medium (1:1, M: L ...
... Few complexes of Transition and Inner transition metals have been synthesized by reacting their metal salts with (5-methoxy- 2- {{(4-Methoxy-3, 5-dimethy- 1-pyridiny) methyl} sulfinyI}-IHbenzimidazole). All the complexes were synthesized in ethanolic medium and refluxed in reaction medium (1:1, M: L ...
BCC-44-4-289-298 - Bulgarian Chemical Communications
... spectra of the Cu(II) complexes and to 1604-1577 cm-1 in the spectra of the Co(II) complexes, which points to the coordination of the υ(C=N) nitrogen to the Cu(II) and the Co(II) ion [26-28]. The FT-IR spectra of the free ligands are characterized by the appearance of a band at 3275 cm-1 for L1H2, 3 ...
... spectra of the Cu(II) complexes and to 1604-1577 cm-1 in the spectra of the Co(II) complexes, which points to the coordination of the υ(C=N) nitrogen to the Cu(II) and the Co(II) ion [26-28]. The FT-IR spectra of the free ligands are characterized by the appearance of a band at 3275 cm-1 for L1H2, 3 ...
AQA A2 CHEMISTRY TRANSITION METALS BOOKLET OF PAST
... Octahedral and tetrahedral complex ions are produced by the reaction of transition metal ions with ligands which form co-ordinate bonds with the transition metal ion. Define the term ligand and explain what is meant by the term co-ordinate bond. ...
... Octahedral and tetrahedral complex ions are produced by the reaction of transition metal ions with ligands which form co-ordinate bonds with the transition metal ion. Define the term ligand and explain what is meant by the term co-ordinate bond. ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... demand in coordination chemistry.Recent advances in technology have now made microwave energy a more efficient means of heating reactions. Chemical transformations that took hours, or even days, to complete their organic reaction, can now be accomplished in minutes. Microwave irradiation is well kno ...
... demand in coordination chemistry.Recent advances in technology have now made microwave energy a more efficient means of heating reactions. Chemical transformations that took hours, or even days, to complete their organic reaction, can now be accomplished in minutes. Microwave irradiation is well kno ...
Chapter 18 - Aldehydes and Ketones
... New Reactions of Aldehydes and Ketones • Nucleophilic Additions We have just reviewed the addition of Grignard reagents to carbonyl. In fact, this reaction is not limited to those reagents. Many other nucleophiles can add to aldehydes and ketones. These nucleophiles can be charged or not, and the n ...
... New Reactions of Aldehydes and Ketones • Nucleophilic Additions We have just reviewed the addition of Grignard reagents to carbonyl. In fact, this reaction is not limited to those reagents. Many other nucleophiles can add to aldehydes and ketones. These nucleophiles can be charged or not, and the n ...
Regents Unit 15b: Aldehydes, Ketones, Carboxylic Acids, & Esters
... • Contain –COOH group. • H is bonded to O. Hydrogen bonding occurs. Leads to increases in boiling point over corresponding alkane. • Also can form hydrogen bonds with water so the smaller acids are pretty soluble. ...
... • Contain –COOH group. • H is bonded to O. Hydrogen bonding occurs. Leads to increases in boiling point over corresponding alkane. • Also can form hydrogen bonds with water so the smaller acids are pretty soluble. ...
Template Synthesis and Characterization of Macrocyclic Complexes
... of isatin and amino group of oxalyldihydrazide and formation of macrocyclic Schiff base8 as these band may be assigned due to ν(C=N)9 azomethine nitrogen. The lowering of this band in the complexes indicates the coordination of nitrogen atoms of azomethine groups to metal atom10. The band present in ...
... of isatin and amino group of oxalyldihydrazide and formation of macrocyclic Schiff base8 as these band may be assigned due to ν(C=N)9 azomethine nitrogen. The lowering of this band in the complexes indicates the coordination of nitrogen atoms of azomethine groups to metal atom10. The band present in ...
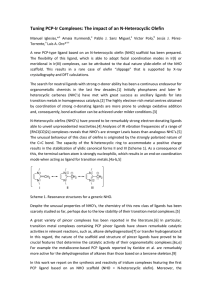
Tuning PCP-Ir Complexes: The impact of an N
... A new PCP-type ligand based on an N-heterocyclic olefin (NHO) scaffold has been prepared. The flexibility of this ligand, which is able to adopt facial coordination modes in Ir(I) or meridional in Ir(III) complexes, can be attributed to the dual nature ylide-olefin of the NHO scaffold. This results ...
... A new PCP-type ligand based on an N-heterocyclic olefin (NHO) scaffold has been prepared. The flexibility of this ligand, which is able to adopt facial coordination modes in Ir(I) or meridional in Ir(III) complexes, can be attributed to the dual nature ylide-olefin of the NHO scaffold. This results ...
Preparation and Characterization of Some
... )[M2(LH)2]Cl2 with a metal to ligand ratio of 2:2 with M = Co(II) or Cu(II), while Ni(II forms complex [Ni2(LH)2(H2O)2Cl2] with a metal to ligand ratio of 2:2, whereas Zn(II) and Cd(II) form complexes of general formula [M2(LH)(H2O)2Cl2] with a metal to ligand ratio of 2 :1 . All compl ...
... )[M2(LH)2]Cl2 with a metal to ligand ratio of 2:2 with M = Co(II) or Cu(II), while Ni(II forms complex [Ni2(LH)2(H2O)2Cl2] with a metal to ligand ratio of 2:2, whereas Zn(II) and Cd(II) form complexes of general formula [M2(LH)(H2O)2Cl2] with a metal to ligand ratio of 2 :1 . All compl ...
Document
... • In the IUPAC system, carboxylic acids are identified by a suffix added to the parent name of the longest chain with different endings being used depending on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and ...
... • In the IUPAC system, carboxylic acids are identified by a suffix added to the parent name of the longest chain with different endings being used depending on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and ...
Abstracts - Thieme Verlag
... This chapter provides a concise overview of metal-catalyzed additions to alkenes that involve carbon monoxide and another nucleophilic species, such as water or an alcohol. This is an important area of research in terms of several commodity chemical targets, with many papers devoted to the evolution ...
... This chapter provides a concise overview of metal-catalyzed additions to alkenes that involve carbon monoxide and another nucleophilic species, such as water or an alcohol. This is an important area of research in terms of several commodity chemical targets, with many papers devoted to the evolution ...
Carboxylic Acids and the Acidity of the O—H Bond
... • In the IUPAC system, carboxylic acids are identified by a suffix added to the parent name of the longest chain with different endings being used depending on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and ...
... • In the IUPAC system, carboxylic acids are identified by a suffix added to the parent name of the longest chain with different endings being used depending on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and ...
Document
... Pd(0) and Pd(II) are both capable of interacting with unsaturated systems such as alkenes or alkynes via p-bonding. The two types of complexes are however different in nature. Pd(0) is highly electron-rich and back-donates to the ligand (Pd L), whereas Pd(II) is electrophilic, and its main interac ...
... Pd(0) and Pd(II) are both capable of interacting with unsaturated systems such as alkenes or alkynes via p-bonding. The two types of complexes are however different in nature. Pd(0) is highly electron-rich and back-donates to the ligand (Pd L), whereas Pd(II) is electrophilic, and its main interac ...
Synthesis of first row transition metal carboxylate complexes by ring
... core in the complex is compensated by an uncoordinated 2,5-dicarbomethoxybenzene 1,4-dicarboxylato dianion. The complex has five coordinated distorted square pyramidal geometries around the copper(II) ions. Further to this, the complex is obtained in a solvated form with 2,5-dicarbomethoxy benzene 1 ...
... core in the complex is compensated by an uncoordinated 2,5-dicarbomethoxybenzene 1,4-dicarboxylato dianion. The complex has five coordinated distorted square pyramidal geometries around the copper(II) ions. Further to this, the complex is obtained in a solvated form with 2,5-dicarbomethoxy benzene 1 ...
CHEM 202_ Part 2
... Carbonyl group is stabilized by adjacent alkyl groups (e-donor), so ketone is more stable than aldehyde. Steric effect also play a role in the relative reactivities of aldehydes and ketones. ...
... Carbonyl group is stabilized by adjacent alkyl groups (e-donor), so ketone is more stable than aldehyde. Steric effect also play a role in the relative reactivities of aldehydes and ketones. ...
193 - Wayne State Chemistry
... of substituents at the carbon atoms. However, application of such complexes as CVD precursors requires monomeric species to achieve the highest possible volatility. Monomeric metal complexes with η2-pz ligands are known for lanthanide and actinide complexes,4,5 but there is only one example of a d-b ...
... of substituents at the carbon atoms. However, application of such complexes as CVD precursors requires monomeric species to achieve the highest possible volatility. Monomeric metal complexes with η2-pz ligands are known for lanthanide and actinide complexes,4,5 but there is only one example of a d-b ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.