
Supported Homogeneous Catalysts
... soluble in the liquidphase reactant, have received a great deal of attention, although they have so far found only limited industrial use, chiefly because of the di$culty of their separation from the reaction products. More recently an intermediate type, made by attaching the active metal complex to ...
... soluble in the liquidphase reactant, have received a great deal of attention, although they have so far found only limited industrial use, chiefly because of the di$culty of their separation from the reaction products. More recently an intermediate type, made by attaching the active metal complex to ...
(Lecture(25) - MSU Chemistry
... ligands'(on'z'axis)'than'for'the'equatorial'ligands'(on'the'x'and'y'axes).'This'change'in' geometry'from'the'octahedral'geometry'typically'observed'for'transition'metals'with'a' coordination'number'of'6'is'called'tetragonal.distortion.' ...
... ligands'(on'z'axis)'than'for'the'equatorial'ligands'(on'the'x'and'y'axes).'This'change'in' geometry'from'the'octahedral'geometry'typically'observed'for'transition'metals'with'a' coordination'number'of'6'is'called'tetragonal.distortion.' ...
Chapter 12: IR Spectroscopy and Mass Spectrometry
... remember about IR spectra is that it is a tool to help determine key functional groups. As such, you should never look to the right of 1600cm-1 (fingerprint region) of an IR spectrum. ...
... remember about IR spectra is that it is a tool to help determine key functional groups. As such, you should never look to the right of 1600cm-1 (fingerprint region) of an IR spectrum. ...
Lecture notes for chapter 6
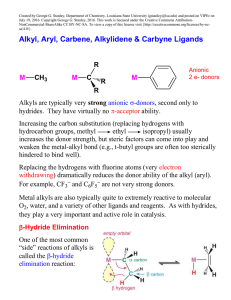
... higher metals with a d6 or d8 electron count (sometimes d4). 4) Schrock alkylidenes as dianionic 4 e- donor ligands. Typically group 4 or 5 metals with d0 electron counts. Also later transition metals in high oxidation states (d0, d2, or d4). Of course, in order to do method 3 or 4, you have to real ...
... higher metals with a d6 or d8 electron count (sometimes d4). 4) Schrock alkylidenes as dianionic 4 e- donor ligands. Typically group 4 or 5 metals with d0 electron counts. Also later transition metals in high oxidation states (d0, d2, or d4). Of course, in order to do method 3 or 4, you have to real ...
I. ALDEHYDES AND KETONES Carbonyl compounds are
... As an example of the use of an aldol reaction in synthesis, consider the preparation of 2-ethyl-2hexenal. This molecule is an unsaturated aldehyde. We can make these by the dehydration of hydroxy aldehydes in aqueous acid. The required hydroxy aldehyde, in turn can be prepared by the aldol c ...
... As an example of the use of an aldol reaction in synthesis, consider the preparation of 2-ethyl-2hexenal. This molecule is an unsaturated aldehyde. We can make these by the dehydration of hydroxy aldehydes in aqueous acid. The required hydroxy aldehyde, in turn can be prepared by the aldol c ...
Chapter 20 reactions of carbonyls
... [1] Convert the OH group into another functional group that does not interfere with the desired reaction. • This new blocking group is called a protecting group, and the reaction that creates it is called “protection”. [2] Carry out the desired reaction. [3] Remove the protecting group. • This react ...
... [1] Convert the OH group into another functional group that does not interfere with the desired reaction. • This new blocking group is called a protecting group, and the reaction that creates it is called “protection”. [2] Carry out the desired reaction. [3] Remove the protecting group. • This react ...
Group 13 and 14 Group 14: Carbon
... • Synthesized by laser ablation of C/M surfaces • M includes Sc, Y, La typically in C80, C82, C84 ...
... • Synthesized by laser ablation of C/M surfaces • M includes Sc, Y, La typically in C80, C82, C84 ...
Coordination Chemistry III: Tanabe
... n empty orbitals (n “holes”) have the same symmetry as dn octahedral configurations: ...
... n empty orbitals (n “holes”) have the same symmetry as dn octahedral configurations: ...
Document
... are carried out with a metal catalyst. • A second way is to add two protons and two electrons to a substrate—that is, H2 = 2H+ + 2e-. Reductions of this sort use alkali metals as a source of electrons, and liquid ammonia as a source of ...
... are carried out with a metal catalyst. • A second way is to add two protons and two electrons to a substrate—that is, H2 = 2H+ + 2e-. Reductions of this sort use alkali metals as a source of electrons, and liquid ammonia as a source of ...
Synthesis and DNA-Binding of Transition Metal Complexes with 3,4
... the ∆ values fall in the range of 174-156 cm-1, indicating a bidentate coordination mode of the carboxylato group17. Abroad band in the range of 3442-3415 cm-1 shows that water molecules is existed in the complexes. Two absorption band at around 931-927 cm-1 attributed to HOH stretching and at 616-6 ...
... the ∆ values fall in the range of 174-156 cm-1, indicating a bidentate coordination mode of the carboxylato group17. Abroad band in the range of 3442-3415 cm-1 shows that water molecules is existed in the complexes. Two absorption band at around 931-927 cm-1 attributed to HOH stretching and at 616-6 ...
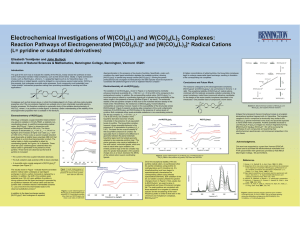
Electrochemical Investigations of W(CO) (L) and W(CO) (L) Complexes:
... the axial carbon monoxide ligands, which are trans to each other upon oxidation; the twisted pyridine rings of the bis complex may allow for greater delocalization of the positive charge and, as a result, decrease the affinity of the radical cation toward coordinating Figure 3: Cyclic voltammogram o ...
... the axial carbon monoxide ligands, which are trans to each other upon oxidation; the twisted pyridine rings of the bis complex may allow for greater delocalization of the positive charge and, as a result, decrease the affinity of the radical cation toward coordinating Figure 3: Cyclic voltammogram o ...
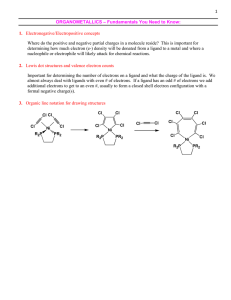
tutorials 1-5
... 10. The magnetic moment of an octahedral complex of cobalt [CoL6]SO4 is 3.8 BM. Draw the CFT diagram of the complex and choose the correct ligand L from the list provided. [ CN-, NH3, H2O, Cl-] 11. Calculate the approximate magnetic moments of the following octahedral complexes using crystal field t ...
... 10. The magnetic moment of an octahedral complex of cobalt [CoL6]SO4 is 3.8 BM. Draw the CFT diagram of the complex and choose the correct ligand L from the list provided. [ CN-, NH3, H2O, Cl-] 11. Calculate the approximate magnetic moments of the following octahedral complexes using crystal field t ...
m4 carbonyl
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
M - Chemistry
... At this point we will consider metal-metal (M-M) bonds to be simple covalent bonds with each metal contributing 1e- to the bond. Most M-M bonded complexes are symmetrical, which means that one only has to e- count one metal center. If the metals are different, however, one does need to figure out ea ...
... At this point we will consider metal-metal (M-M) bonds to be simple covalent bonds with each metal contributing 1e- to the bond. Most M-M bonded complexes are symmetrical, which means that one only has to e- count one metal center. If the metals are different, however, one does need to figure out ea ...
1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 11 Apr
... Several observables identify this as an authentic dihydrogen complex vs. a dihydride: • d(H—H) = 0.84 Å (as measured from neutron diffraction). This distance is near the bond distance of free H2, d(H—H) = 0.7414 Å. • a symmetric H2 vibration is observed, ν(H—H) = 2,690 cm–1, as compared to ν(H—H) = ...
... Several observables identify this as an authentic dihydrogen complex vs. a dihydride: • d(H—H) = 0.84 Å (as measured from neutron diffraction). This distance is near the bond distance of free H2, d(H—H) = 0.7414 Å. • a symmetric H2 vibration is observed, ν(H—H) = 2,690 cm–1, as compared to ν(H—H) = ...
Consider the diamagnetic complex, [Os(NH3)5(CO)]
... [Os(NH3)6]3+/2+. Briefly explain these results in terms of the natures of the bonding of the CO and NH3 ligands to metal ions. The redox potentials are for the reduction processes: [Os(NH3)5(CO)]3+ + e- Æ [Os(NH3)5(CO)]2+ [Os(NH3)6]3+ + e- Æ [Os(NH3)6]2+ As described in above in part (ii), the Os-CO ...
... [Os(NH3)6]3+/2+. Briefly explain these results in terms of the natures of the bonding of the CO and NH3 ligands to metal ions. The redox potentials are for the reduction processes: [Os(NH3)5(CO)]3+ + e- Æ [Os(NH3)5(CO)]2+ [Os(NH3)6]3+ + e- Æ [Os(NH3)6]2+ As described in above in part (ii), the Os-CO ...
Solubility of alkali metals in non
... Figure 3. Scheme of the apparatus for preparation of metal solutions. Small pieces of the metal (K, Na; ca 2-3g) are put into part A, melted and degassed a. elevated temperature under the continuous flow of the inert gas (argon). The distillation is carried out in high vacuum (10" torr). The walls o ...
... Figure 3. Scheme of the apparatus for preparation of metal solutions. Small pieces of the metal (K, Na; ca 2-3g) are put into part A, melted and degassed a. elevated temperature under the continuous flow of the inert gas (argon). The distillation is carried out in high vacuum (10" torr). The walls o ...
Organomet-2
... A. Ionic compounds with very electropositive metals e.g. NaC6H5 Behaves as Na+ C6H5- - an insoluble and pyrophoric (i.e. spontaneously inflame in air) solid. Not much practical use due to its insolubility. B. Polar covalent compounds e.g. Grignard reagents CH3--Mg+-X. They are reactive and are oft ...
... A. Ionic compounds with very electropositive metals e.g. NaC6H5 Behaves as Na+ C6H5- - an insoluble and pyrophoric (i.e. spontaneously inflame in air) solid. Not much practical use due to its insolubility. B. Polar covalent compounds e.g. Grignard reagents CH3--Mg+-X. They are reactive and are oft ...
Chemistry B11 Chapters 16-18 Amines, aldehydes, ketones and
... charge and oxygen obtains the partial negative charge). 3. There is only the dipole-dipole interaction between the molecules and there is no possibility for hydrogen bonding. 4. They have lower boiling points than amines and alcohols. 5. They are soluble in water (form hydrogen bonds with water - th ...
... charge and oxygen obtains the partial negative charge). 3. There is only the dipole-dipole interaction between the molecules and there is no possibility for hydrogen bonding. 4. They have lower boiling points than amines and alcohols. 5. They are soluble in water (form hydrogen bonds with water - th ...
07.3 - Reactions in aqueous solutions
... # FIGURE 4.11 Familiar corrosion products. (a) A green coating forms when copper is oxidized. (b) Rust forms when iron corrodes. (c) A black tarnish forms as silver corrodes. ...
... # FIGURE 4.11 Familiar corrosion products. (a) A green coating forms when copper is oxidized. (b) Rust forms when iron corrodes. (c) A black tarnish forms as silver corrodes. ...
Synthesis and Characterization of Dinuclear Metal Complexes
... metal atom through the azomethine nitrogen atom (Sallomi and Al-Shaheen, 1994). Another important band which appeared at 1243 and 1236 cm−1 due to ν(C-O) stretching in the free ligand is shifted to the lower field in the prepared complexes. This is usually indicates that the (C-O) groups of the liga ...
... metal atom through the azomethine nitrogen atom (Sallomi and Al-Shaheen, 1994). Another important band which appeared at 1243 and 1236 cm−1 due to ν(C-O) stretching in the free ligand is shifted to the lower field in the prepared complexes. This is usually indicates that the (C-O) groups of the liga ...
OSU Spectr 08
... complexes with other amino acids have also been shown to be ZW. Many dimer complexes M+2Trp2 are readily formed in the electrospray. What the conformation of the second ligand in the Ba+2Trp2 case, and what is the conformation of both ligands in the general dimer case? ...
... complexes with other amino acids have also been shown to be ZW. Many dimer complexes M+2Trp2 are readily formed in the electrospray. What the conformation of the second ligand in the Ba+2Trp2 case, and what is the conformation of both ligands in the general dimer case? ...
Reaction mechanism of Coordination Complexes
... Reaction mechanism of Coordination Complexes Complexes are classified as Inert and Labile ( kinetic stability) depending on their reactivity. According to Henry Taube, a Nobel Laureate, the definition is ...
... Reaction mechanism of Coordination Complexes Complexes are classified as Inert and Labile ( kinetic stability) depending on their reactivity. According to Henry Taube, a Nobel Laureate, the definition is ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.











![Consider the diamagnetic complex, [Os(NH3)5(CO)]](http://s1.studyres.com/store/data/006728268_1-5aa3fd1c498fab420574702fa8076d3a-300x300.png)








