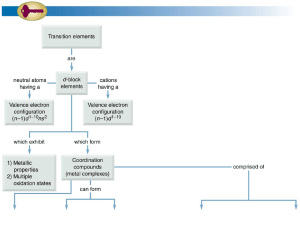
Coordination Compounds Coordination
... carnallite (KCl. MgCl2. 6H2 O), Mohr’s salt [FeSO 4. (NH4) 2 SO 4. 6H2 O] Complex ions do not dissociate further to give simpler ions. For example, [Fe(CN) 6]4– , [Fe(C2 O 4) 3 ]3– Ligands: Ions or molecules bound to the central metal atom or ion in the coordination entity Didentate – ...
... carnallite (KCl. MgCl2. 6H2 O), Mohr’s salt [FeSO 4. (NH4) 2 SO 4. 6H2 O] Complex ions do not dissociate further to give simpler ions. For example, [Fe(CN) 6]4– , [Fe(C2 O 4) 3 ]3– Ligands: Ions or molecules bound to the central metal atom or ion in the coordination entity Didentate – ...
Coordination Compounds
... attached groups called ligands. Coordination sphere is the area of the central atom and ligands. Coordination number is the number of points where ligands attach. Complex ion is a complex that carries a charge. Coordination compound is substance with one or more complexes. ...
... attached groups called ligands. Coordination sphere is the area of the central atom and ligands. Coordination number is the number of points where ligands attach. Complex ion is a complex that carries a charge. Coordination compound is substance with one or more complexes. ...
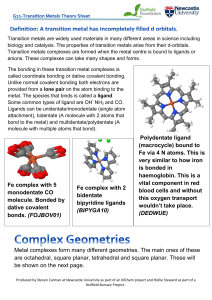
Polydentate ligand (macrocycle) bound to Fe via 4 N atoms. This is
... Octahedral: One of the most common geometries is octahedral which comes from it having 8 faces. There are 6 atoms around the atom in total, 4 atoms in one plane with the other 2 above and below this plane. All bond angles should be 90o. (FUBYIK) Octahedral molecules can form isomers such as cis/tra ...
... Octahedral: One of the most common geometries is octahedral which comes from it having 8 faces. There are 6 atoms around the atom in total, 4 atoms in one plane with the other 2 above and below this plane. All bond angles should be 90o. (FUBYIK) Octahedral molecules can form isomers such as cis/tra ...
Nugget
... Investigation of the Lewis Acidic Properties of N-Heterocyclic Carbene Ligands and Their Implications for Catalysis Colin D. Abernethy, Department of Chemistry, Keene State College Keene, NH 03435 Cyclopentadienyl complexes of vanadium chlorides, which also contain N-heterocyclic carbene (NHC) ligan ...
... Investigation of the Lewis Acidic Properties of N-Heterocyclic Carbene Ligands and Their Implications for Catalysis Colin D. Abernethy, Department of Chemistry, Keene State College Keene, NH 03435 Cyclopentadienyl complexes of vanadium chlorides, which also contain N-heterocyclic carbene (NHC) ligan ...
Transition Metals
... in space than are the 3d orbitals. They have a higher quantum number n and the have fewer nodes (0 or 1) versus 2 for the d orbitals. ...
... in space than are the 3d orbitals. They have a higher quantum number n and the have fewer nodes (0 or 1) versus 2 for the d orbitals. ...
The catalytic hydrogenolysis of esters to the two corresponding
... with phosphines. Upon coordination to transition metals, these ligands give the resulting metal complex stability and electron density that is needed for the formation of hydride species. Hydride species are important intermediates in the catalytic cycle of hydrogenolysis and hydrogenation reactions ...
... with phosphines. Upon coordination to transition metals, these ligands give the resulting metal complex stability and electron density that is needed for the formation of hydride species. Hydride species are important intermediates in the catalytic cycle of hydrogenolysis and hydrogenation reactions ...
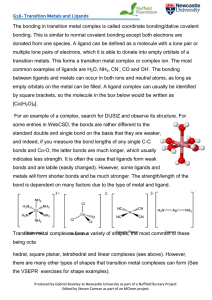
The bonding in transition metal complex is called coordinate
... donated from one species. A ligand can be defined as a molecule with a lone pair or multiple lone pairs of electrons, which it is able to donate into empty orbitals of a transition metals. This forms a transition metal complex or complex ion. The most common examples of ligands are H2O, NH3, CN-, CO ...
... donated from one species. A ligand can be defined as a molecule with a lone pair or multiple lone pairs of electrons, which it is able to donate into empty orbitals of a transition metals. This forms a transition metal complex or complex ion. The most common examples of ligands are H2O, NH3, CN-, CO ...
transition metals - Department of Chemistry | Oregon State University
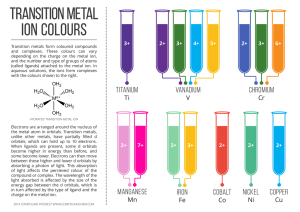
... high density high melting and boiling points often form colored compounds solid at room temperature (except mercury) • form complex ions • often paramagnetic (Wikipedia) ...
... high density high melting and boiling points often form colored compounds solid at room temperature (except mercury) • form complex ions • often paramagnetic (Wikipedia) ...
Chemistry of Coordination Compounds
... model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. In both cases, the coordination geometry is octahedral around Co. ...
... model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. In both cases, the coordination geometry is octahedral around Co. ...
CH 23 HW
... 9. The types of constitutional isomerism (coordination and linkage) and stereoisomerism (geometric and optical) of coordination compounds (§23.3) 10. How valence bond theory uses hybridization to account for the shapes of octahedral, square planar, and ...
... 9. The types of constitutional isomerism (coordination and linkage) and stereoisomerism (geometric and optical) of coordination compounds (§23.3) 10. How valence bond theory uses hybridization to account for the shapes of octahedral, square planar, and ...
EXAMINING THE IMPACT OF LIGAND BASICITY ON THE REACTIVITY OF TRANSITION METAL SYSTEMS THROUGH COMPUTATIONAL METHODS
... The electron-donating properties of supporting ligands can have a dramatic impact on the properties and observed reactivity of transition metal complexes. Indeed, gaining the ability to “tune” the properties of metal complexes is a primary goal in inorganic and organometallic chemistry. Unfortunatel ...
... The electron-donating properties of supporting ligands can have a dramatic impact on the properties and observed reactivity of transition metal complexes. Indeed, gaining the ability to “tune” the properties of metal complexes is a primary goal in inorganic and organometallic chemistry. Unfortunatel ...
Chapter 21 Transition Metals and Coordination Chemistry
... A coordination compound consists of a complex ion along with one or more counterions which are anions or cations required to produce a compound with no net charge Square brackets are placed around the complex ion to indicate the composition of the complex ion Counterions are shown outside the bracke ...
... A coordination compound consists of a complex ion along with one or more counterions which are anions or cations required to produce a compound with no net charge Square brackets are placed around the complex ion to indicate the composition of the complex ion Counterions are shown outside the bracke ...
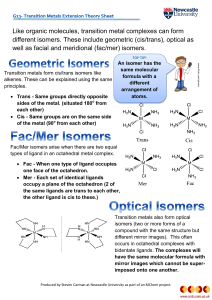
Like organic molecules, transition metal complexes can form
... compound with the same structure but different mirror images). This often occurs in octahedral complexes with bidentate ligands. The complexes will have the same molecular formula with mirror images which cannot be superimposed onto one another. Produced by Steven Carman at Newcastle University as p ...
... compound with the same structure but different mirror images). This often occurs in octahedral complexes with bidentate ligands. The complexes will have the same molecular formula with mirror images which cannot be superimposed onto one another. Produced by Steven Carman at Newcastle University as p ...
Powerpoint - mvhs
... ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. In both cases, the coordination geometry is octahedral around Co. ...
... ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. In both cases, the coordination geometry is octahedral around Co. ...
Coordination Compounds [Compatibility Mode]
... ion and two Cl- are the counter ions. • There are 5 NH3 ligands and one Cl- ligand that create the complex ion. • When dissolved in water, the solid behaves like any ionic solid (the cations and anions dissociate) ...
... ion and two Cl- are the counter ions. • There are 5 NH3 ligands and one Cl- ligand that create the complex ion. • When dissolved in water, the solid behaves like any ionic solid (the cations and anions dissociate) ...
Transition metals and complex ions
... Explain the term ligand in terms of coordinate bonding. Describe and use the terms: complex ion and coordination number. State and give examples of complexes with six-fold coordination with an octahedral shape. ...
... Explain the term ligand in terms of coordinate bonding. Describe and use the terms: complex ion and coordination number. State and give examples of complexes with six-fold coordination with an octahedral shape. ...
Coordination Compounds
... • Complex ion: can be cation or anion; contains the transition metal and usually is in brackets. • Ligands: neutral molecule or ion having a lone pair of electrons that forms a coordinate covalent bond to a metal ion; usually dissociate when aqueous. Ligands in the spectrochemical series show which ...
... • Complex ion: can be cation or anion; contains the transition metal and usually is in brackets. • Ligands: neutral molecule or ion having a lone pair of electrons that forms a coordinate covalent bond to a metal ion; usually dissociate when aqueous. Ligands in the spectrochemical series show which ...
Key Concepts PowerPoint
... Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the +x and +y axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex. ...
... Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the +x and +y axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex. ...
Chapter 24: Transition Metals Coordination Compounds Part 1
... The covalently attached ions or molecules are called ligands. The ligand atom which is directly connected to the metal atom/ion is called the ligand donor atom or just the donor atom. So in the above, ammonia is the ligand. If the complex is an ion, like the above, it is written in brackets as shown ...
... The covalently attached ions or molecules are called ligands. The ligand atom which is directly connected to the metal atom/ion is called the ligand donor atom or just the donor atom. So in the above, ammonia is the ligand. If the complex is an ion, like the above, it is written in brackets as shown ...
Chapter 24: Transition Metals Coordination Compounds Part 2
... Chapter 24: Transition Metals Coordination Compounds Part 2 Transition Metals ...
... Chapter 24: Transition Metals Coordination Compounds Part 2 Transition Metals ...
Abstract Coordination Chemistry of Tetra(pyrazolyl)
... ligands. It was found that the silver(I) complexes display unique solution behavior in which the solid state structure is not retained. Finally, given the importance of oxyferryl species in mediating C-H bond oxidation reactions; the chemistry of iron(II) complexes of the newly synthesized ligands w ...
... ligands. It was found that the silver(I) complexes display unique solution behavior in which the solid state structure is not retained. Finally, given the importance of oxyferryl species in mediating C-H bond oxidation reactions; the chemistry of iron(II) complexes of the newly synthesized ligands w ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.











![Coordination Compounds [Compatibility Mode]](http://s1.studyres.com/store/data/000678035_1-c20c75fd4abb97d3ba4a0b0fce26e10b-300x300.png)