Coordination Chemistry I: Structures and Isomers
... • Anionic ligands are given an ‘o’ suffix. Neutral ligands retain the usual name. – Coordianted water is called ‘aqua’. ...
... • Anionic ligands are given an ‘o’ suffix. Neutral ligands retain the usual name. – Coordianted water is called ‘aqua’. ...
lect4b
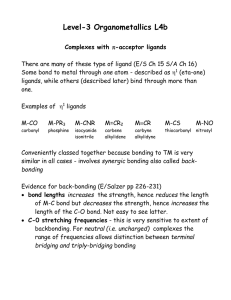
... Level-3 Organometallics L4b Complexes with -acceptor ligands There are many of these type of ligand (E/S Ch 15 S/A Ch 16) Some bond to metal through one atom - described as 1 (eta-one) ligands, while others (described later) bind through more than one. Examples of 1 ligands M-CO ...
... Level-3 Organometallics L4b Complexes with -acceptor ligands There are many of these type of ligand (E/S Ch 15 S/A Ch 16) Some bond to metal through one atom - described as 1 (eta-one) ligands, while others (described later) bind through more than one. Examples of 1 ligands M-CO ...
Crystal Field Theory www.AssignmentPoint.com Crystal Field
... dz2 and dx2-y2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experience less repulsion. The three lower-energy orbitals are collectively referred to as t2g, and the two higher-energy orbitals as eg. (These labels are based on the t ...
... dz2 and dx2-y2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experience less repulsion. The three lower-energy orbitals are collectively referred to as t2g, and the two higher-energy orbitals as eg. (These labels are based on the t ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 Part-A
... Organometallic compounds are formed by transition metal ions in the low oxidation state. Comment. Cl- occupies the lower end of the spectrochemical series, yet the 10Dq value in [RuCl6]3- is comparable to that of [Ru(H2O)6]2+. Offer a reasonable explanation. Jahn-Teller distortion arising from the e ...
... Organometallic compounds are formed by transition metal ions in the low oxidation state. Comment. Cl- occupies the lower end of the spectrochemical series, yet the 10Dq value in [RuCl6]3- is comparable to that of [Ru(H2O)6]2+. Offer a reasonable explanation. Jahn-Teller distortion arising from the e ...
The complex in biological systems Plan 1. Definition of complex
... The centerpiece of the complex compound takes complexing - positively charged ion (usually a metal d elements) 2. Around complexing ligands are (adenty), is ions of opposite sign or neutral molecule CI-, CN-, H2O0; NH30 number that shows how many ligands holds complexing, called the coordination nu ...
... The centerpiece of the complex compound takes complexing - positively charged ion (usually a metal d elements) 2. Around complexing ligands are (adenty), is ions of opposite sign or neutral molecule CI-, CN-, H2O0; NH30 number that shows how many ligands holds complexing, called the coordination nu ...
transition metals
... DRAW the resonance structures in order to explain! Remember that a linkage isomer can bond to the metal ion from two distinctly different places on the molecule. And in order to have a bond – you need electrons!!! a. NO2-1 b. SO2 c. NO3-1 ...
... DRAW the resonance structures in order to explain! Remember that a linkage isomer can bond to the metal ion from two distinctly different places on the molecule. And in order to have a bond – you need electrons!!! a. NO2-1 b. SO2 c. NO3-1 ...
crystal field theory, spectrochemical series, high spin
... I− < Br− < S−2 < SCN− < Cl− < NO2− < F− < OH− < H2O < NCS− < py < bipy < NO2− < CN− < CO The ligands interact weakly : - weak field ligands e.g. I−, Br−, S−2, SCN−, Cl− The ligands interact strongly: - strong field ligands e.g. NO2− ,CN− ,CO ...
... I− < Br− < S−2 < SCN− < Cl− < NO2− < F− < OH− < H2O < NCS− < py < bipy < NO2− < CN− < CO The ligands interact weakly : - weak field ligands e.g. I−, Br−, S−2, SCN−, Cl− The ligands interact strongly: - strong field ligands e.g. NO2− ,CN− ,CO ...
CBSE Class 12 Chemistry notes and questions for
... Counter ions: The ionisable groups written outside the square bracket. Ex- K+ in K4[Fe(CN)6] OR 3Cl- in [Co(NH3)6]Cl3 vii) Coordination Polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central metal atom/ion. They are commonly Octahedral, Square-planar or Te ...
... Counter ions: The ionisable groups written outside the square bracket. Ex- K+ in K4[Fe(CN)6] OR 3Cl- in [Co(NH3)6]Cl3 vii) Coordination Polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central metal atom/ion. They are commonly Octahedral, Square-planar or Te ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 14. Explain the working principle of dye-sensitized solar cells. 15. The epr spectrum of bis(salicylaldimine)copper(II) consists of four sets of eleven lines each. Explain the spectral features. Substantiate your interpretation with experimental evidences. 16. How do you differentiate [Co(en)3]3+ an ...
... 14. Explain the working principle of dye-sensitized solar cells. 15. The epr spectrum of bis(salicylaldimine)copper(II) consists of four sets of eleven lines each. Explain the spectral features. Substantiate your interpretation with experimental evidences. 16. How do you differentiate [Co(en)3]3+ an ...
role of the ancillary ligands on the coordination modes
... Nuovi farmaci inorganici in oncologia agreement with the formation of a single reaction product. In the 1H NMR spectrum, the presence of one of the NH resonances at δ 10.6 suggests that platination of the neutral nucleobase occurs at the N(4) atom, as result of a tautomeric shift of one of the exocy ...
... Nuovi farmaci inorganici in oncologia agreement with the formation of a single reaction product. In the 1H NMR spectrum, the presence of one of the NH resonances at δ 10.6 suggests that platination of the neutral nucleobase occurs at the N(4) atom, as result of a tautomeric shift of one of the exocy ...
03 Complexation equilibrium
... The term complex in chemistry is usually used to describe molecules or ensembles formed by the combination of ligands and metal ions. The molecules or ions that surround the central metal ion in a coordination compound are called ligands, and the atoms that are attached directly to the metal ar ...
... The term complex in chemistry is usually used to describe molecules or ensembles formed by the combination of ligands and metal ions. The molecules or ions that surround the central metal ion in a coordination compound are called ligands, and the atoms that are attached directly to the metal ar ...
Isomerism of coordination compounds
... D.F. Shriver, P.W. Atkins, C.H. Langford, Inorganic Chemistry, Oxford University Press, 1994 * Bo Xin, Summary to the Seminar of the Lecture Advanced Inorganic Chemistry, Siegen 08/09 ...
... D.F. Shriver, P.W. Atkins, C.H. Langford, Inorganic Chemistry, Oxford University Press, 1994 * Bo Xin, Summary to the Seminar of the Lecture Advanced Inorganic Chemistry, Siegen 08/09 ...
Ch 24 Part 2 PowerPoint
... Square planar complexes can be thought of as octahedral complexes with the two ligands along the z-axis removed. As a consequence the four planar ligands are drawn in closer towards the metal. Relative to the octahedral field, the dz2 orbital is greatly lowered in energy, the dyz, and dxz orbita ...
... Square planar complexes can be thought of as octahedral complexes with the two ligands along the z-axis removed. As a consequence the four planar ligands are drawn in closer towards the metal. Relative to the octahedral field, the dz2 orbital is greatly lowered in energy, the dyz, and dxz orbita ...
Inorganic Chemistry review sheet Exam #3 Ch. 9 Lewis acids (e
... Tetragonal Distortion (Oh) (elongation or compression yield different splitting) Td can also undergo distortions Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy ther ...
... Tetragonal Distortion (Oh) (elongation or compression yield different splitting) Td can also undergo distortions Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy ther ...
metal ion
... If the ligand is a negative ion: ‘ide’ is dropped and replaced with ‘o’ e.g. chloride becomes chloro , cyanide becomes cyano; ‘ate’ and ‘ite’ change to ‘ato’ or ‘ito’ e.g. oxalate becomes oxalato, nitrite becomes nitrito. ...
... If the ligand is a negative ion: ‘ide’ is dropped and replaced with ‘o’ e.g. chloride becomes chloro , cyanide becomes cyano; ‘ate’ and ‘ite’ change to ‘ato’ or ‘ito’ e.g. oxalate becomes oxalato, nitrite becomes nitrito. ...
Critical Thinking Worksheet 9
... If this process leads to unpaired electrons, the complex is paramagnetic and is attracted towards magnetic field. If there are no unpaired electrons, the complex is diamagnetic and is weakly repelled by magnets. ...
... If this process leads to unpaired electrons, the complex is paramagnetic and is attracted towards magnetic field. If there are no unpaired electrons, the complex is diamagnetic and is weakly repelled by magnets. ...
15.2 COMPLEX FORMATION And THE SHAPE OF COMPLEX IONS
... Ligands which donate 2 lone pairs per ligand and are said to be bidentate. Multidentate ligands have more than one lone pair which they can bond to a transition metal. ...
... Ligands which donate 2 lone pairs per ligand and are said to be bidentate. Multidentate ligands have more than one lone pair which they can bond to a transition metal. ...
Bidentate & multidentate ligands File
... a) Explain the term “bidentate ligand”. b) What is the coordination number of the [Fe(C2O4)3]3complex. c) Use your answer to part (b) to suggest what shape the [Fe(C2O4)3]3- complex is and draw it. ...
... a) Explain the term “bidentate ligand”. b) What is the coordination number of the [Fe(C2O4)3]3complex. c) Use your answer to part (b) to suggest what shape the [Fe(C2O4)3]3- complex is and draw it. ...
Chem 106 Thurs 4-21-2011 Ch. 22: Transition Metals 1. Review
... based more on ionic attractive forces. Larger cations with smaller positive charge tend to form more stable compounds with large anions with valence electrons in larger, more diffuse orbitals. These compounds are based more on covalent bonding interactions. ...
... based more on ionic attractive forces. Larger cations with smaller positive charge tend to form more stable compounds with large anions with valence electrons in larger, more diffuse orbitals. These compounds are based more on covalent bonding interactions. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.