Information regarding naming of Complex Ions Reference Sources: Naming Complex Ions
... For splitting of d orbitals due to ligands around central metal ion see: http://www.chemguide.co.uk/inorganic/complexions/colour2.html ...
... For splitting of d orbitals due to ligands around central metal ion see: http://www.chemguide.co.uk/inorganic/complexions/colour2.html ...
e-nomenclature-of-coordination-compounds-take-home-2
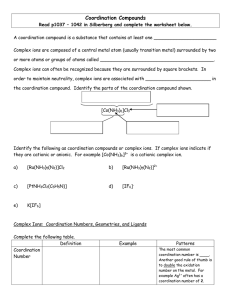
... Coordination Compounds Read p1037 – 1042 in Silberberg and complete the worksheet below. A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of ...
... Coordination Compounds Read p1037 – 1042 in Silberberg and complete the worksheet below. A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of ...
Lecture`23 - MSU Chemistry - Michigan State University
... [Co(NH3)4Cl2]+)[tetraamminedichlorocobalt(III)]) ...
... [Co(NH3)4Cl2]+)[tetraamminedichlorocobalt(III)]) ...
File
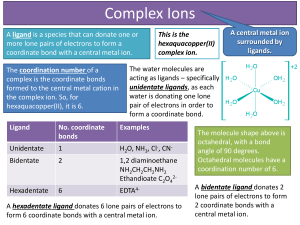
... A ligand is a species that can donate one or more lone pairs of electrons to form a coordinate bond with a central metal ion. The coordination number of a complex is the coordinate bonds formed to the central metal cation in the complex ion. So, for hexaquacopper(II), it is 6. ...
... A ligand is a species that can donate one or more lone pairs of electrons to form a coordinate bond with a central metal ion. The coordination number of a complex is the coordinate bonds formed to the central metal cation in the complex ion. So, for hexaquacopper(II), it is 6. ...
Transition Metal Chemistry
... Metals due to their small size and positive charge, will attract –ve species and form dative bonds (coordinate) with species that are electron rich (ligands). Lewis acid – electron pair acceptor (M+) Lewis base – electron pair donator (L) Alfred Werner – known as the father of coordinate chemistry p ...
... Metals due to their small size and positive charge, will attract –ve species and form dative bonds (coordinate) with species that are electron rich (ligands). Lewis acid – electron pair acceptor (M+) Lewis base – electron pair donator (L) Alfred Werner – known as the father of coordinate chemistry p ...
Complex Ions - Frankie Guglieri
... Complex Ions What is a complex metal ion? A complex ion has a metal ion at its center with a number of other molecules or ions surrounding it. These can be considered to be attached to the central ion by co-ordinate covalent bonds. (In some cases, the bonding is actually more complicated than that.) ...
... Complex Ions What is a complex metal ion? A complex ion has a metal ion at its center with a number of other molecules or ions surrounding it. These can be considered to be attached to the central ion by co-ordinate covalent bonds. (In some cases, the bonding is actually more complicated than that.) ...
Coordination Chemistry
... from one chemical species is donated to an empty orbital on another chemical species to form the new bond. This is different from a covalent bond because both electrons come from one atom or molecule but are shared as in a typical covalent bond. Unlike an ionic bond, a coordinate covalent bond does ...
... from one chemical species is donated to an empty orbital on another chemical species to form the new bond. This is different from a covalent bond because both electrons come from one atom or molecule but are shared as in a typical covalent bond. Unlike an ionic bond, a coordinate covalent bond does ...
Geometric and Electronic Structures of Complexes
... Cu(II) is the most acidic metal ion, Zn(II) is next (although it is the hardest ion in the series) ...
... Cu(II) is the most acidic metal ion, Zn(II) is next (although it is the hardest ion in the series) ...
Structural Preferences of N-Substituted Monosaccharide Derivatives
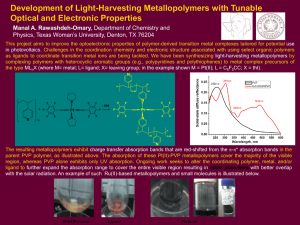
... Physics, Texas Woman’s University, Denton, TX 76204 This project aims to improve the optoelectronic properties of polymer-derived transition metal complexes tailored for potential use in photovoltaics. Challenges in the coordination chemistry and electronic structure associated with using select org ...
... Physics, Texas Woman’s University, Denton, TX 76204 This project aims to improve the optoelectronic properties of polymer-derived transition metal complexes tailored for potential use in photovoltaics. Challenges in the coordination chemistry and electronic structure associated with using select org ...
Transition Metal Chemistry - WordPress.com
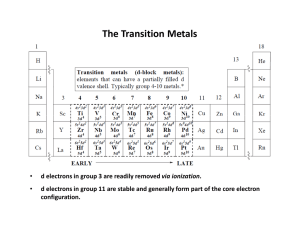
... • Transition metals are defined as metallic elements with an incomplete d sub-shell in at least one of their ions. • Form positive (+) ions by losing electrons. • These electrons come from the 4s sub-shell first, then from the 3d sub-shell: Fe atom: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d6 Fe2+ ion: ...
... • Transition metals are defined as metallic elements with an incomplete d sub-shell in at least one of their ions. • Form positive (+) ions by losing electrons. • These electrons come from the 4s sub-shell first, then from the 3d sub-shell: Fe atom: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d6 Fe2+ ion: ...
TRANSITION METALS - Pennsylvania State University
... unaffected by a magnetic field no unpaired electrons Paramagnetic: influenced by a magnetic field unpaired electrons Transition metals and their compounds are often paramagnetic Have unpaired d-electrons Eg. Ti2+ Mn2+ ...
... unaffected by a magnetic field no unpaired electrons Paramagnetic: influenced by a magnetic field unpaired electrons Transition metals and their compounds are often paramagnetic Have unpaired d-electrons Eg. Ti2+ Mn2+ ...
RULES FOR NAMING COORDINATION COMPLEXES The name of
... J"rgensen's Chain Theory J~rgensen's chain theory links ammonia molecules in metal compounds similar to the linking of carbon units in hydrocarbons. Like carbon, each metal center is thought to have a fixed valence (valency being defined as the number of bonds formed by the atom of interest), with ...
... J"rgensen's Chain Theory J~rgensen's chain theory links ammonia molecules in metal compounds similar to the linking of carbon units in hydrocarbons. Like carbon, each metal center is thought to have a fixed valence (valency being defined as the number of bonds formed by the atom of interest), with ...
Chemistry of Coordination Compounds
... Ex: transition metals such as Cu2+ (blue) and Fe3+ (orange) ...
... Ex: transition metals such as Cu2+ (blue) and Fe3+ (orange) ...
Synthesis and Crystal Structure of Nicotinamide Cobalt (II) Complexes
... the body, known as pellagra disease. Victims of pellagra show unusually high serum and urinary copper levels (1). The nicotinic acid derivative N,N-diethylnicotinamide (DENA) is an important respiratory stimulant (2). Transition metal complexes with biochemical molecules show interesting physical an ...
... the body, known as pellagra disease. Victims of pellagra show unusually high serum and urinary copper levels (1). The nicotinic acid derivative N,N-diethylnicotinamide (DENA) is an important respiratory stimulant (2). Transition metal complexes with biochemical molecules show interesting physical an ...
Lanthanum has only one important oxidation state in aqueous
... • Same ligands, arranged differently around TM • cis- and trans- ...
... • Same ligands, arranged differently around TM • cis- and trans- ...
Chapter 24
... general formula is MX3Y3 called fac-mer (short for facial and meridian). An example is Co(NH3)3Cl3. ...
... general formula is MX3Y3 called fac-mer (short for facial and meridian). An example is Co(NH3)3Cl3. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.