notes on Bohr and the hydrogen spectrum
... The first question in the series is a simple one, and one that you have probably asked yourself in the past. Why is grass green? Why is blood red? Why are things coloured? In many cases, this is because molecules absorb or emit only certain frequencies. Atomic gases are the simplest cases, and here ...
... The first question in the series is a simple one, and one that you have probably asked yourself in the past. Why is grass green? Why is blood red? Why are things coloured? In many cases, this is because molecules absorb or emit only certain frequencies. Atomic gases are the simplest cases, and here ...
Chapter 27
... • The diffracted radiation is very intense in the directions that correspond to constructive interference from waves reflected from the layers of the crystal ...
... • The diffracted radiation is very intense in the directions that correspond to constructive interference from waves reflected from the layers of the crystal ...
Atomic Structure
... A dental hygienist uses x-rays (l= 1.00A) to take a series of dental radiographs while the patient listens to a radio station (l = 325 cm) and looks out the window at the blue sky (l= 473 nm). What is the frequency (in s-1) of the electromagnetic radiation from each source? (Assume that the radiatio ...
... A dental hygienist uses x-rays (l= 1.00A) to take a series of dental radiographs while the patient listens to a radio station (l = 325 cm) and looks out the window at the blue sky (l= 473 nm). What is the frequency (in s-1) of the electromagnetic radiation from each source? (Assume that the radiatio ...
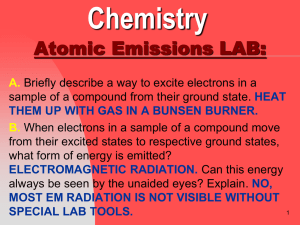
Atomic Emissions LAB Questions
... THEM UP WITH GAS IN A BUNSEN BURNER. B. When electrons in a sample of a compound move from their excited states to respective ground states, what form of energy is emitted? ELECTROMAGNETIC RADIATION. Can this energy always be seen by the unaided eyes? Explain. NO, MOST EM RADIATION IS NOT VISIBLE WI ...
... THEM UP WITH GAS IN A BUNSEN BURNER. B. When electrons in a sample of a compound move from their excited states to respective ground states, what form of energy is emitted? ELECTROMAGNETIC RADIATION. Can this energy always be seen by the unaided eyes? Explain. NO, MOST EM RADIATION IS NOT VISIBLE WI ...
Chapter 7 Radiation from Charged Particle Interaction with Matter
... A key reason is that since the photon energy emitted extends from zero up to the electron’s incident energy, we have to deal with photons having energies comparable to the electron rest mass or more, and hence carrying away momentum that is critical in the scattering process. One way to think about ...
... A key reason is that since the photon energy emitted extends from zero up to the electron’s incident energy, we have to deal with photons having energies comparable to the electron rest mass or more, and hence carrying away momentum that is critical in the scattering process. One way to think about ...
Interaction of Radiation with Matter
... radiation or EMR) is a form of energy emitted and absorbed by charged particles which exhibits wave-like behavior as it travels through space. EMR has both electric and magnetic ...
... radiation or EMR) is a form of energy emitted and absorbed by charged particles which exhibits wave-like behavior as it travels through space. EMR has both electric and magnetic ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
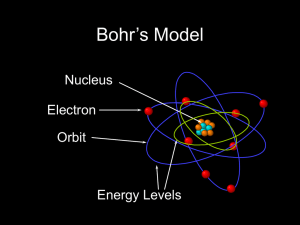
... Atoms, Molecules, Ions – fundamentals of elements o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an ...
... Atoms, Molecules, Ions – fundamentals of elements o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an ...
Liad Elmelech 7.1-7.3 The Nature of Light, Atomic Spectroscopy
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
Ch1-8 Brown and LeMay Review
... On your own paper, answer the following questions. 1. Consider the molecules PF3 and PF5 A) Draw the Lewis electron-dot structures for both. B) Predict the geometries and bond angles for each. 2. Explain each of the following observations using principles of atomic structure. A) Potassium has a lowe ...
... On your own paper, answer the following questions. 1. Consider the molecules PF3 and PF5 A) Draw the Lewis electron-dot structures for both. B) Predict the geometries and bond angles for each. 2. Explain each of the following observations using principles of atomic structure. A) Potassium has a lowe ...
here
... Hotter objects emit more photons, so hotter objects are brighter objects Energy emitted per unit surface area ~ T4 Double an object’s temperature, and it emits 16 times as much energy! (16 = 24) Triple the temperature, and it emits 81 times as much energy!! ...
... Hotter objects emit more photons, so hotter objects are brighter objects Energy emitted per unit surface area ~ T4 Double an object’s temperature, and it emits 16 times as much energy! (16 = 24) Triple the temperature, and it emits 81 times as much energy!! ...
21Sc , 48 22Ti , 50 22Ti , 50
... NOTE: This ‘practice exam’ contains more than questions than the real final. 1. The wavelength of light emitted from a green laser pointer is 5.32 × 102 nm. What is the wavelength in meters? 2. What is the correct answer, with correct significant figures, to the following expression: (18 + 95) × 0.0 ...
... NOTE: This ‘practice exam’ contains more than questions than the real final. 1. The wavelength of light emitted from a green laser pointer is 5.32 × 102 nm. What is the wavelength in meters? 2. What is the correct answer, with correct significant figures, to the following expression: (18 + 95) × 0.0 ...
The Development of a New Atomic Model
... • It is type of electromagnetic radiation • Forms part of the electromagnetic spectrum • Significant wavelike motion characterized by wavelength and frequency – Wavelength (λ) is the distance between corresponding points on adjacent waves. – Frequency (v) is the number of waves that pass a specific ...
... • It is type of electromagnetic radiation • Forms part of the electromagnetic spectrum • Significant wavelike motion characterized by wavelength and frequency – Wavelength (λ) is the distance between corresponding points on adjacent waves. – Frequency (v) is the number of waves that pass a specific ...
Quantum Theory Chapter 27
... • Recall that electromagnetic radiation theory states, the more intense the radiation, regardless of the frequency, the stronger the electric and the resulting magnetic field. Thus for low intensity radiation the electrons would need to absorb radiation for a long period of time to reach the thresh ...
... • Recall that electromagnetic radiation theory states, the more intense the radiation, regardless of the frequency, the stronger the electric and the resulting magnetic field. Thus for low intensity radiation the electrons would need to absorb radiation for a long period of time to reach the thresh ...
Quantum Mechanics I. Introduction Just before 1900, the classical
... results, had to assume that each oscillator could only have an integral number of units of energy, rather than have any arbitrary amount. In other words, the energy of each oscillator was quantized. D. Planck saw this quantization as purely a mathematical trick to get the theory to fit the data. How ...
... results, had to assume that each oscillator could only have an integral number of units of energy, rather than have any arbitrary amount. In other words, the energy of each oscillator was quantized. D. Planck saw this quantization as purely a mathematical trick to get the theory to fit the data. How ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.