PPT - hrsbstaff.ednet.ns.ca
... It is impossible to predict when a specific nucleus will decay You can describe the probability of decay The concept of half life is used with radioactive decay: the time required for half of the sample to decay Using the half life equation, it is possible to determine how much of a sample w ...
... It is impossible to predict when a specific nucleus will decay You can describe the probability of decay The concept of half life is used with radioactive decay: the time required for half of the sample to decay Using the half life equation, it is possible to determine how much of a sample w ...
Unit 9 - Chemical Quantities: The Mole
... Molar Mass • Molar Mass (MM) – Mass of one mole of a substance – Unit is grams/mole • g/mole or g/mol – Same as molecular mass with different unit • molecular mass of water = 18.02 amu • Molar Mass of water = 18.02 g/mol ...
... Molar Mass • Molar Mass (MM) – Mass of one mole of a substance – Unit is grams/mole • g/mole or g/mol – Same as molecular mass with different unit • molecular mass of water = 18.02 amu • Molar Mass of water = 18.02 g/mol ...
empirical formula
... ratio of atoms that always exists for that compound Example: Water – H2O Always 2 H atoms to 1 O atom ...
... ratio of atoms that always exists for that compound Example: Water – H2O Always 2 H atoms to 1 O atom ...
explanation
... mass of 0.5 MeV. It is clear from this figures that the mass of the hydrogen atom is the mass of the nucleus while the dimension of the atom is determined by the radius at which we can still find the electron charge. This means that the atom is basically empty being the dimension of the nucleus a fa ...
... mass of 0.5 MeV. It is clear from this figures that the mass of the hydrogen atom is the mass of the nucleus while the dimension of the atom is determined by the radius at which we can still find the electron charge. This means that the atom is basically empty being the dimension of the nucleus a fa ...
Electronics
... • Planck himself was not entirely happy with this idea, but this was in fact the birth of modern physics. B The Bohr's Theory In 1913 Niels Henrik Bohr published his new theory of the atoms constitution. Just like Rutherford he assumed that electrons rotate around the nucleus. But had the three comp ...
... • Planck himself was not entirely happy with this idea, but this was in fact the birth of modern physics. B The Bohr's Theory In 1913 Niels Henrik Bohr published his new theory of the atoms constitution. Just like Rutherford he assumed that electrons rotate around the nucleus. But had the three comp ...
Conductivities and transmission coefficients of ultra-thin disordered metallic films B. J.
... As is well established, a proper quantum mechanical description of electron transport in disordered systems is based on the linearised von Neumann equation for the density matrix. In the lowest order of perturbation theory, the diagonal elements of the density matrix satisfy a Boltzmann-type equatio ...
... As is well established, a proper quantum mechanical description of electron transport in disordered systems is based on the linearised von Neumann equation for the density matrix. In the lowest order of perturbation theory, the diagonal elements of the density matrix satisfy a Boltzmann-type equatio ...
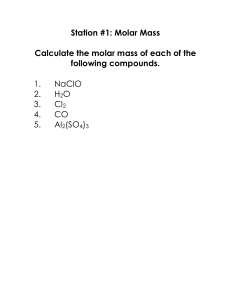
Station #1: Molar Mass
... Station #5: Update Binder Update your binder so you are all ready for the binder check on Monday! ...
... Station #5: Update Binder Update your binder so you are all ready for the binder check on Monday! ...
atomicspectra1-2
... Alkalis and Alkali-like Spectra • In the central field approximation there exists no angular-momentum coupling between a closed subshell and an electron outside the subshell, since the net spin and orbital angular momenta of the subshell are both zero. nlj quantum numbers are appropriate for a sing ...
... Alkalis and Alkali-like Spectra • In the central field approximation there exists no angular-momentum coupling between a closed subshell and an electron outside the subshell, since the net spin and orbital angular momenta of the subshell are both zero. nlj quantum numbers are appropriate for a sing ...
Lecture 5
... • The angular momentum of the e- is quantized • The attraction between p and e- provides the centripetal acceleration ...
... • The angular momentum of the e- is quantized • The attraction between p and e- provides the centripetal acceleration ...
No Slide Title
... • Chemists have shown that the electron arrangement of an atom is related to its CHEMICAL PROPERTIES (how it reacts). • It has been found that elements in the same group of the periodic table have very similar properties. • Looking at the electron arrangement of elements within a group it can be see ...
... • Chemists have shown that the electron arrangement of an atom is related to its CHEMICAL PROPERTIES (how it reacts). • It has been found that elements in the same group of the periodic table have very similar properties. • Looking at the electron arrangement of elements within a group it can be see ...
9. Time-dependent Perturbation Theory
... and, by implication, the Hamiltonian, is not an explicit function of time. This allowed us to solve the time-dependent Schrödinger equation by separation of variables, i.e., Ψ(r, t) = ψ(r)e−iEt/~. We now want to treat transitions between quantum states, which are driven by a ...
... and, by implication, the Hamiltonian, is not an explicit function of time. This allowed us to solve the time-dependent Schrödinger equation by separation of variables, i.e., Ψ(r, t) = ψ(r)e−iEt/~. We now want to treat transitions between quantum states, which are driven by a ...
Chapter 3
... finding an electron at various locations around the nucleus of. An atomic orbitals is represented pictorially as a region of space in which there is a high probably of finding an electron. ...
... finding an electron at various locations around the nucleus of. An atomic orbitals is represented pictorially as a region of space in which there is a high probably of finding an electron. ...
Chapter 38
... a) The circumference of an electron’s orbit in an atom is an integer multiple of the electron’s wavelength. b) The positive charge within an atom is concentrated within a very small volume within an atom. c) A low pressure monatomic gas can be made to emit electromagnetic waves viewed as a series of ...
... a) The circumference of an electron’s orbit in an atom is an integer multiple of the electron’s wavelength. b) The positive charge within an atom is concentrated within a very small volume within an atom. c) A low pressure monatomic gas can be made to emit electromagnetic waves viewed as a series of ...
on the behaviour of atoms in an electromagnetic wa ve field
... As well known, the properties of the atoms cannot be accoun.ted for on the basis of the classical theory of electrons. Still they exhibit in many respects a great similarity with the properties which, on the classical theory, systems consisting of small · electrically charged particles would possess ...
... As well known, the properties of the atoms cannot be accoun.ted for on the basis of the classical theory of electrons. Still they exhibit in many respects a great similarity with the properties which, on the classical theory, systems consisting of small · electrically charged particles would possess ...
Document
... • Determine momenta from path in the magnetic field (wire chambers and focal plane detectors) • Electrons have high energies ...
... • Determine momenta from path in the magnetic field (wire chambers and focal plane detectors) • Electrons have high energies ...
naming-and-formulas-chem-1-ab
... The second element uses the suffix “-ide”. Prefixes are added to the name of each element to indicate the NUMBER of atoms of the element in the molecule. (If the first element’s prefix is mono-, it will be dropped. For example, monocarbon dioxide (CO2) is simply called carbon ...
... The second element uses the suffix “-ide”. Prefixes are added to the name of each element to indicate the NUMBER of atoms of the element in the molecule. (If the first element’s prefix is mono-, it will be dropped. For example, monocarbon dioxide (CO2) is simply called carbon ...
13 Black-body radiation and Planck`s formula
... occured in 1913. In that year, a young (and later, the great) Danish physicist Niels Bohr related Planck’s hypothesis of discretness of radiation with two then-unexplainable phenomena inside the atom: the atom’s stability and radiation spectra emitted by atoms. A couple years before that, in 1911, E ...
... occured in 1913. In that year, a young (and later, the great) Danish physicist Niels Bohr related Planck’s hypothesis of discretness of radiation with two then-unexplainable phenomena inside the atom: the atom’s stability and radiation spectra emitted by atoms. A couple years before that, in 1911, E ...
Name: Date: Chemistry 1 – Midterm Review Sheet Unit 1 – Scientific
... Early Atomic Theory 6. The scientist whose alpha-particle scattering experiment led him to conclude that the nucleus of an atom contains a dense center of positive charge is a. J. J. Thomson b. Lord Kelvin c. Ernest Rutherford d. William Thomson e. James Chadwick Atomic Structure 7 . How many proton ...
... Early Atomic Theory 6. The scientist whose alpha-particle scattering experiment led him to conclude that the nucleus of an atom contains a dense center of positive charge is a. J. J. Thomson b. Lord Kelvin c. Ernest Rutherford d. William Thomson e. James Chadwick Atomic Structure 7 . How many proton ...
Name Date Class Period ______
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
Learning material
... inside of an atom. Recall that even up to the beginning of the 20th century atoms were regarded as indivisible. Thompson was led to a different conclusion by his discovery of the electron as a constituent of atoms. Thomson’s model distributed the positive and negative charge uniformly through the at ...
... inside of an atom. Recall that even up to the beginning of the 20th century atoms were regarded as indivisible. Thompson was led to a different conclusion by his discovery of the electron as a constituent of atoms. Thomson’s model distributed the positive and negative charge uniformly through the at ...
The Nature of the Atom The Nature of the Atom
... • Suppose an evacuated glass tube is filled with hydrogen (=atom made of 1 proton and 1 electron) at low pressure. If a sufficiently highly voltage is applied between metal electrodes in the tube, an electric current flows. The gas then emits light. • Analyzing the emitted light with a spectrograph, ...
... • Suppose an evacuated glass tube is filled with hydrogen (=atom made of 1 proton and 1 electron) at low pressure. If a sufficiently highly voltage is applied between metal electrodes in the tube, an electric current flows. The gas then emits light. • Analyzing the emitted light with a spectrograph, ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.