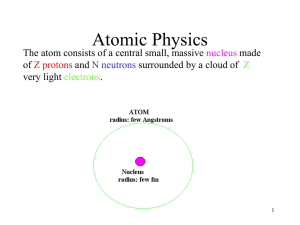
Atomic
... description of a particle's behavior, provided it was not moving at a speed near that of light. In spite of this success, the very meaning of the waves remained unclear. Schrödinger believed that the intensity of the wave at a point in space represented the 'amount' of the electron that was present ...
... description of a particle's behavior, provided it was not moving at a speed near that of light. In spite of this success, the very meaning of the waves remained unclear. Schrödinger believed that the intensity of the wave at a point in space represented the 'amount' of the electron that was present ...
Chemistry Chapter 5 notes (10/20, PDF)
... s, is the ___________ quantum number and is used to show the orientation of an electron in an orbital. By convention the first electron is said to spin clockwise and is assigned a value of +1/2; the second electron in an orbital must have counterclockwise spin and is assigned a value of -1/2. The co ...
... s, is the ___________ quantum number and is used to show the orientation of an electron in an orbital. By convention the first electron is said to spin clockwise and is assigned a value of +1/2; the second electron in an orbital must have counterclockwise spin and is assigned a value of -1/2. The co ...
Advanced electronic bonding and how these affect molecular shapes
... Electronic configuration naming • As we know, electrons occupy certain energy levels around the atom. • These energy levels are called shells. • Electrons jump to higher energy levels when provided with energy, but will automatically drop back down to the lowest energy level possible. • These energ ...
... Electronic configuration naming • As we know, electrons occupy certain energy levels around the atom. • These energy levels are called shells. • Electrons jump to higher energy levels when provided with energy, but will automatically drop back down to the lowest energy level possible. • These energ ...
Chemical Nomenclature (ionic compounds)
... non-metal. The metal portion will always appear first in the name and formula. b) The total number of electrons lost by the metal atom(s) must equal the total number of electrons gained by the nonmetal atom(s). (The charge left after an atom gains or loses electrons is called its valence.) c) The sy ...
... non-metal. The metal portion will always appear first in the name and formula. b) The total number of electrons lost by the metal atom(s) must equal the total number of electrons gained by the nonmetal atom(s). (The charge left after an atom gains or loses electrons is called its valence.) c) The sy ...
Interstellar Masers - Physics
... emission is proportional to N1 or N2 , respectively, so if N1 > N2 , then absorption will occur faster than stimulated emission, and no maser can be produced.4 As a second case, if N1 = N2 , the rate of photons produced by stimulated emission will be equal to the rate of photons absorbed, so, again, ...
... emission is proportional to N1 or N2 , respectively, so if N1 > N2 , then absorption will occur faster than stimulated emission, and no maser can be produced.4 As a second case, if N1 = N2 , the rate of photons produced by stimulated emission will be equal to the rate of photons absorbed, so, again, ...
High energy universe – Satellite missions
... Associated with acceleration of charged particles is the emission of radiation. The standard radiation processes are the bremsstrahlung, synchrotron and the inverse Compton and the Raman scattering. Often a combination of these processes is used to account for the observed radiation spectrum as for ...
... Associated with acceleration of charged particles is the emission of radiation. The standard radiation processes are the bremsstrahlung, synchrotron and the inverse Compton and the Raman scattering. Often a combination of these processes is used to account for the observed radiation spectrum as for ...
27-4 Photons Carry Momentum
... Thus, after the collision, the photon has lower energy, which means its frequency is lower while its wavelength !/ is higher. The photon’s direction changes by an angle #. Applying energy conservation to this situation gives one equation, while applying momentum conservation in two dimensions gives ...
... Thus, after the collision, the photon has lower energy, which means its frequency is lower while its wavelength !/ is higher. The photon’s direction changes by an angle #. Applying energy conservation to this situation gives one equation, while applying momentum conservation in two dimensions gives ...
1 - INFN-LNF
... The plasma regimes experienced by the Laplace spacecraft are the solar wind and the magnetosphere of Jupiter, and varies through the mission as Laplace travels from Earth to Jupiter. In general, the energetic radiation environment consists of magnetically trapped charged particles, solar protons and ...
... The plasma regimes experienced by the Laplace spacecraft are the solar wind and the magnetosphere of Jupiter, and varies through the mission as Laplace travels from Earth to Jupiter. In general, the energetic radiation environment consists of magnetically trapped charged particles, solar protons and ...
Lesson 13: Nuclear Propulsion Basics
... • Stronger than the electrostatic force binding electrons to the nucleus or repelling protons from one another • Limited in range to a few x 10-15 m ...
... • Stronger than the electrostatic force binding electrons to the nucleus or repelling protons from one another • Limited in range to a few x 10-15 m ...
11.6 Nuclear Radiation
... Alpha particles have about 8000 times the mass of an electron. Alpha radiation is strongly ionising as the large, slow moving alpha particles are very likely to collide with atoms as they pass through a substance. (An ion is a charged atom that has fewer or more electrons than normal, so that the po ...
... Alpha particles have about 8000 times the mass of an electron. Alpha radiation is strongly ionising as the large, slow moving alpha particles are very likely to collide with atoms as they pass through a substance. (An ion is a charged atom that has fewer or more electrons than normal, so that the po ...
Lecture 7: Light Waves Newton`s Laws of Motion (1666) Newton`s
... •If we know both wavelengths, then we can measure the relative speed v … ...
... •If we know both wavelengths, then we can measure the relative speed v … ...
Chapter 5: Electrons in Atoms 1 Section 5.1: Light and Quantized
... Limits an electron’s energy to certain values Doesn’t describe the electron’s path around the nucleus The solution to Schrödinger’s wave equation is known as a wave function o wave function is related to the probability of finding the electrons in a particular volume of space around the nucleus o el ...
... Limits an electron’s energy to certain values Doesn’t describe the electron’s path around the nucleus The solution to Schrödinger’s wave equation is known as a wave function o wave function is related to the probability of finding the electrons in a particular volume of space around the nucleus o el ...
Atomic and Nuclear Physics
... calculated that the diameter of the gold nucleus could not be larger than 10-14 m. This diagram is not to scale. With a 1 mm diameter nucleus the diameter of the atom would have to be 10000 mm or 10 m! The nucleus is like a pea at the centre of a football pitch. ...
... calculated that the diameter of the gold nucleus could not be larger than 10-14 m. This diagram is not to scale. With a 1 mm diameter nucleus the diameter of the atom would have to be 10000 mm or 10 m! The nucleus is like a pea at the centre of a football pitch. ...
Slide 1 - Southwest High School
... find a series of individual lines, called a line spectrum. This is the line spectrum of hydrogen. ...
... find a series of individual lines, called a line spectrum. This is the line spectrum of hydrogen. ...
Chapter 5 - Cloudfront.net
... • Bohr also proposed electrons orbit around nucleus • Electrons orbit in fixed energy levels. • Electrons can move up and down energy levels. • Energy is involved in this movement ...
... • Bohr also proposed electrons orbit around nucleus • Electrons orbit in fixed energy levels. • Electrons can move up and down energy levels. • Energy is involved in this movement ...
PPT - hrsbstaff.ednet.ns.ca
... It is impossible to predict when a specific nucleus will decay You can describe the probability of decay The concept of half life is used with radioactive decay: the time required for half of the sample to decay Using the half life equation, it is possible to determine how much of a sample w ...
... It is impossible to predict when a specific nucleus will decay You can describe the probability of decay The concept of half life is used with radioactive decay: the time required for half of the sample to decay Using the half life equation, it is possible to determine how much of a sample w ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.