The angular momentum quantum number
... As shown in the top row of the figure (a), there are two s orbitals — one for energy level 1 (1s) and the other for energy level 2 (2s). The s orbitals are spherical with the nucleus at the center. Notice that the 2s orbital is larger in diameter than the 1s orbital. In large atoms, the 1s orbital ...
... As shown in the top row of the figure (a), there are two s orbitals — one for energy level 1 (1s) and the other for energy level 2 (2s). The s orbitals are spherical with the nucleus at the center. Notice that the 2s orbital is larger in diameter than the 1s orbital. In large atoms, the 1s orbital ...
Review Sheet
... 9. Describe the bonding in a given compound (this includes: which orbitals overlap to form each bond, what type(s) of bonds are formed, and how many electrons are in each bond). Also, know the orbital location of any lone pairs of electrons. 10. Which type of bonds allow for free rotation around the ...
... 9. Describe the bonding in a given compound (this includes: which orbitals overlap to form each bond, what type(s) of bonds are formed, and how many electrons are in each bond). Also, know the orbital location of any lone pairs of electrons. 10. Which type of bonds allow for free rotation around the ...
molecular geometry
... For H2, we begin with the two 1s atomic orbitals on the two H atoms. There are two ways in which these can be combined, corresponding to two molecular orbitals. One molecular orbital lowers the energy and therefore corresponds to a bonding orbital, while the other molecular orbital raises the ener ...
... For H2, we begin with the two 1s atomic orbitals on the two H atoms. There are two ways in which these can be combined, corresponding to two molecular orbitals. One molecular orbital lowers the energy and therefore corresponds to a bonding orbital, while the other molecular orbital raises the ener ...
Parts of Unit 4 and 5Chp 5-6 – Electrons and
... The angular momentum quantum number (l) can be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (m) can be any ...
... The angular momentum quantum number (l) can be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (m) can be any ...
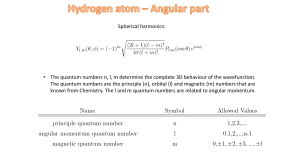
Spherical harmonics: • The quantum numbers n, l, m determine the
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
QUANTUM-MECHANICAL MODEL OF THE ATOM Quantum
... l=0 → spherical shape with nucles at the center → s orbital for H atom's ground state → the electron probability density is highest at the nucleus (Fig. 7.17A) Fig. 7.17B → Because the 2s orbital is larger than the 1s, an electron in 2s spend more time farther from the nucleus than when it occupies ...
... l=0 → spherical shape with nucles at the center → s orbital for H atom's ground state → the electron probability density is highest at the nucleus (Fig. 7.17A) Fig. 7.17B → Because the 2s orbital is larger than the 1s, an electron in 2s spend more time farther from the nucleus than when it occupies ...
The Atom
... ground state quantumanumber De Broglie equation Heisenberg uncertainty principle quantum mechanical model of the atom atomic orbital principal quantum number principal energy level energy sublevel electron configuration Aufbau principle Hund’s Rule ...
... ground state quantumanumber De Broglie equation Heisenberg uncertainty principle quantum mechanical model of the atom atomic orbital principal quantum number principal energy level energy sublevel electron configuration Aufbau principle Hund’s Rule ...
Quantum Number, n. - Lyndhurst Schools
... Equation gives rise to ‘Orbitals.’ These orbitals provide the electron density distributed about the nucleus. Orbitals are described by quantum numbers. ...
... Equation gives rise to ‘Orbitals.’ These orbitals provide the electron density distributed about the nucleus. Orbitals are described by quantum numbers. ...
electron configuration
... Example: carbon’s outer two electrons are in the 2p sublevel where there are three available atomic orbitals: px, py, pz – If the first electron enters px, the second electron will not pair with it in the same orbital; it will enter one of the available empty orbitals (py or pz) ...
... Example: carbon’s outer two electrons are in the 2p sublevel where there are three available atomic orbitals: px, py, pz – If the first electron enters px, the second electron will not pair with it in the same orbital; it will enter one of the available empty orbitals (py or pz) ...
Inorganic Pharmaceutical Chemistry Hybrid Orbitals Hybridization
... theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Based on the valence bond theory, carbon would only be able to form two covalent bonds, making CH2. However, as you will find out, we know that this is n ...
... theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Based on the valence bond theory, carbon would only be able to form two covalent bonds, making CH2. However, as you will find out, we know that this is n ...
Chapter 2 - UCF Chemistry
... Basic Postulates of Quantum Theory Atoms and molecules can exist only in certain energy states. In each energy state, the atom or molecule has a definite energy. When an atom or molecule changes its energy state, it must emit or absorb just enough energy to bring it to the new energy state (the quan ...
... Basic Postulates of Quantum Theory Atoms and molecules can exist only in certain energy states. In each energy state, the atom or molecule has a definite energy. When an atom or molecule changes its energy state, it must emit or absorb just enough energy to bring it to the new energy state (the quan ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.