SED122 - National Open University of Nigeria
... Unlike metals, non metals do not have characteristic lustre. Many are gases at room temperature and others are solids except bromine which is a red brown liquid at room temperature. Non metals are non-conductors of heat and electricity. They cannot be rolled into sheets or drawn into wires like the ...
... Unlike metals, non metals do not have characteristic lustre. Many are gases at room temperature and others are solids except bromine which is a red brown liquid at room temperature. Non metals are non-conductors of heat and electricity. They cannot be rolled into sheets or drawn into wires like the ...
Chapter 8: Ionic Compounds
... The strong attraction of positive ions and negative ions in an ionic compound results in a crystal lattice. A crystal lattice is a three-dimensional geometric arrangement of particles. In a crystal lattice, each positive ion is surrounded by negative ions and each negative ion is surrounded by posit ...
... The strong attraction of positive ions and negative ions in an ionic compound results in a crystal lattice. A crystal lattice is a three-dimensional geometric arrangement of particles. In a crystal lattice, each positive ion is surrounded by negative ions and each negative ion is surrounded by posit ...
CHE 128 Autumn 2011 Specific Objectives – Exam 1 A periodic
... Identify an exothermic reaction (heat is released/lost) Identify an endothermic reaction (heat is absorbed/added) Identify an element Recall the boiling point of water Convert units of temperature (˚C, ˚F, K) Calculate the specific heat capacity of a substance – equation will be given Compare specif ...
... Identify an exothermic reaction (heat is released/lost) Identify an endothermic reaction (heat is absorbed/added) Identify an element Recall the boiling point of water Convert units of temperature (˚C, ˚F, K) Calculate the specific heat capacity of a substance – equation will be given Compare specif ...
Solid State Physics – Lecture notes
... For example, in 1D, we could use the basis of a diatomic molecule, such as HCl. We then specify a line of dots (the lattice sites), and structure (the shape of the HCl molecule). We then arrage the HCl along the lattice, where each lattice site is the same as every other; the HCl could be imagined a ...
... For example, in 1D, we could use the basis of a diatomic molecule, such as HCl. We then specify a line of dots (the lattice sites), and structure (the shape of the HCl molecule). We then arrage the HCl along the lattice, where each lattice site is the same as every other; the HCl could be imagined a ...
Chemistry 1 Lectures
... ionic compounds consist of a combination of cations and anions • the formula is always the same as the empirical formula • the sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero ...
... ionic compounds consist of a combination of cations and anions • the formula is always the same as the empirical formula • the sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero ...
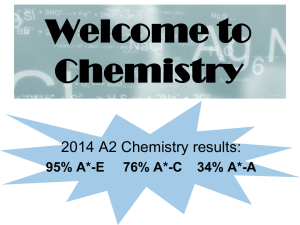
Welcome to Chemistry
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
Chapter 2
... explains the mass laws Definite composition Atoms are combined in compounds in specific ratios ...
... explains the mass laws Definite composition Atoms are combined in compounds in specific ratios ...
Supplemental Informaton
... for the structure. i. Given a chemical formula, ABn, A is generally the central atom and B flanks the A atom. i.e., NH3, NCl3, NO2. In these examples, N is central in each structure. ii. H and F are never central atoms even if their elemental symbol is first, i.e., H2O. ...
... for the structure. i. Given a chemical formula, ABn, A is generally the central atom and B flanks the A atom. i.e., NH3, NCl3, NO2. In these examples, N is central in each structure. ii. H and F are never central atoms even if their elemental symbol is first, i.e., H2O. ...
15anespp
... • leaded petrol must not pass through the catalyst as the lead deposits on the catalyst’s surface and “poisons” it, thus blocking sites for reactions to take place. ...
... • leaded petrol must not pass through the catalyst as the lead deposits on the catalyst’s surface and “poisons” it, thus blocking sites for reactions to take place. ...
CHAPTER 9 CHEMICAL BONDING I
... The octet rule, formulated by Lewis, states that an atom other than hydrogen tends to form bonds until it is surrounded by eight valence electrons. In other words, a covalent bond forms when there are not enough electrons for each individual atom to have a complete octet. By sharing electrons in a c ...
... The octet rule, formulated by Lewis, states that an atom other than hydrogen tends to form bonds until it is surrounded by eight valence electrons. In other words, a covalent bond forms when there are not enough electrons for each individual atom to have a complete octet. By sharing electrons in a c ...
2007 - Thompson Rivers University
... The contest consists of 25 multiple choice questions. You have 60 minutes to complete the test. All questions are of equal value, there is no particular order to the questions and there is no penalty for incorrect answers. Please answer on the Scantron Answer Sheet. In the top right hand corner of t ...
... The contest consists of 25 multiple choice questions. You have 60 minutes to complete the test. All questions are of equal value, there is no particular order to the questions and there is no penalty for incorrect answers. Please answer on the Scantron Answer Sheet. In the top right hand corner of t ...
bond
... • The Pauli exclusion principle: only two electrons can occupy one atomic orbital and the two electrons have opposite spin • Hund’s rule: electrons will occupy empty degenerated orbitals before pairing up in the same orbital Electrons in inner shells (those below the outermost shell) are called core ...
... • The Pauli exclusion principle: only two electrons can occupy one atomic orbital and the two electrons have opposite spin • Hund’s rule: electrons will occupy empty degenerated orbitals before pairing up in the same orbital Electrons in inner shells (those below the outermost shell) are called core ...
AP Chemistry
... 66. The purpose of weighing the cup and its contents again at CaCl2(s) Ca2+ + 2 Clthe end of the experiment was to For the process of solid calcium chloride dissolving in water, (A) determine the mass of solute that was added. represented above, the entropy change might be expected to (B) determi ...
... 66. The purpose of weighing the cup and its contents again at CaCl2(s) Ca2+ + 2 Clthe end of the experiment was to For the process of solid calcium chloride dissolving in water, (A) determine the mass of solute that was added. represented above, the entropy change might be expected to (B) determi ...
Review 1
... made of silver but does not want it damaged during the analysis. The chemist decides to determine the density, knowing that silver has a density of 10.5 g/ml. The figurine is put into a graduated cylinder that contains 32.6 ml of water. The reading while the figurine is in the water is 60.1 ml. The ...
... made of silver but does not want it damaged during the analysis. The chemist decides to determine the density, knowing that silver has a density of 10.5 g/ml. The figurine is put into a graduated cylinder that contains 32.6 ml of water. The reading while the figurine is in the water is 60.1 ml. The ...
Review Packet - Daigneault Chem.is.try
... 9. Describe 4 properties of metals, and 4 properties of non-metals: 10. Which 2 elements touching the staircase are metals, and not metalloids? 11. Where are elements with similar properties found on the periodic table (in horizontal rows, or in vertical columns?) ...
... 9. Describe 4 properties of metals, and 4 properties of non-metals: 10. Which 2 elements touching the staircase are metals, and not metalloids? 11. Where are elements with similar properties found on the periodic table (in horizontal rows, or in vertical columns?) ...
CHEMISTRY PHYSICAL SETTING Thursday, PS/CHEMISTRY
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
The production and use of metals
... 1. the aluminium oxide has to be molten so that it splits into mobile ions Al3+ and O2-. This takes enormous amounts of energy. Aluminium oxide is an ionic compound. Because of its giant lattice structure it has a very high melting point (efforts to reduce the melting point of aluminium oxide are ma ...
... 1. the aluminium oxide has to be molten so that it splits into mobile ions Al3+ and O2-. This takes enormous amounts of energy. Aluminium oxide is an ionic compound. Because of its giant lattice structure it has a very high melting point (efforts to reduce the melting point of aluminium oxide are ma ...
6 Chemistry of Transition Metals
... exchange reactions, represented by [Cr(NH3)6]3+ or [Co(NH3)6]3+. They have been particularly important in the history of the development of coordination chemistry. [Mo(CO)6], [RhCl6]3-, etc. are also octahedral complexes. In the case of mixed ligands, cis- and trans-[MA4B2] and mer- and fac-[MA3B3] ...
... exchange reactions, represented by [Cr(NH3)6]3+ or [Co(NH3)6]3+. They have been particularly important in the history of the development of coordination chemistry. [Mo(CO)6], [RhCl6]3-, etc. are also octahedral complexes. In the case of mixed ligands, cis- and trans-[MA4B2] and mer- and fac-[MA3B3] ...
Chemistry I Syllabus 2011-2012
... inorganic, kinetic energy, law of conservation of mass, light energy, luster, malleability, melting point, metal, metalloid, molecule, nonmetal, organic, phase change, physical properties, potential energy, pure substance, reactivity with air (oxidation), solute, solution, solvent, strength, sub-let ...
... inorganic, kinetic energy, law of conservation of mass, light energy, luster, malleability, melting point, metal, metalloid, molecule, nonmetal, organic, phase change, physical properties, potential energy, pure substance, reactivity with air (oxidation), solute, solution, solvent, strength, sub-let ...
MATTER-Ch. 3-homogeneous vs. heterogeneous, elements
... Which part of an atom has a mass approximately equal to 1/2000 of the mass of a common hydrogen atom? a. nucleus c. proton b. electron d. electron cloud ____ 26. The mass of a neutron is a. about the same as that of a proton. c. double that of a proton. b. about the same as that of an electron. d. d ...
... Which part of an atom has a mass approximately equal to 1/2000 of the mass of a common hydrogen atom? a. nucleus c. proton b. electron d. electron cloud ____ 26. The mass of a neutron is a. about the same as that of a proton. c. double that of a proton. b. about the same as that of an electron. d. d ...
aq - Wikispaces
... The table on the left gives the eight most commonly used prefixes in the metric system. It also includes five rows that do not have prefixes. The middle row is for the unit: metre, litre, gram, newton, or any other legal metric unit. ...
... The table on the left gives the eight most commonly used prefixes in the metric system. It also includes five rows that do not have prefixes. The middle row is for the unit: metre, litre, gram, newton, or any other legal metric unit. ...
I. scientific notation. – a shorthand that scientists use when dealing
... i.Fahrenheit – bp H2O 212ºF ii.Celsius – bp H2O 100ºC 5 x (ºF – 32)/9 iii.Kelvin - bp H2O 373 K (Celsius + 273) b. the SI unit of heat and energy is the joule (J). [another useful unit is the calorie – the amount of heat required to raise the temperature of 1 g of H2O from 14.5ºC to 15.5ºC. 1 cal = ...
... i.Fahrenheit – bp H2O 212ºF ii.Celsius – bp H2O 100ºC 5 x (ºF – 32)/9 iii.Kelvin - bp H2O 373 K (Celsius + 273) b. the SI unit of heat and energy is the joule (J). [another useful unit is the calorie – the amount of heat required to raise the temperature of 1 g of H2O from 14.5ºC to 15.5ºC. 1 cal = ...
Part 3 Answers Only for Questions, Exercises, and Problems in The
... states, but it is probably a mixture. (c) could be either a pure substance or a mixture because it may be one kind of matter or two or more types of matter with similar appearances. 26. Yes, the terms homogeneous and heterogeneous refer to the macroscopic appearance of a sample. A container filled w ...
... states, but it is probably a mixture. (c) could be either a pure substance or a mixture because it may be one kind of matter or two or more types of matter with similar appearances. 26. Yes, the terms homogeneous and heterogeneous refer to the macroscopic appearance of a sample. A container filled w ...
2002 local exam - Virginia Section
... the lettered choice that best fits the statement for each question and fill in the corresponding block on the answer sheet. You may use a choice more than once, once, or not at all. (A) density (B) equilibrium constant (C) freezing point (D) molarity (E) molecular mass 4. Can be expressed in moles p ...
... the lettered choice that best fits the statement for each question and fill in the corresponding block on the answer sheet. You may use a choice more than once, once, or not at all. (A) density (B) equilibrium constant (C) freezing point (D) molarity (E) molecular mass 4. Can be expressed in moles p ...