GCSE ADDITIONAL CHEMISTRY (C2) REVISION BOOKLET
... ............................ or how quickly the products are ....................... . An example of a chemical reaction is the reaction between calcium carbonate and hydrochloric acid: CaCO3(s) + 2HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) We could measure the rate of this reaction by measuring the .... ...
... ............................ or how quickly the products are ....................... . An example of a chemical reaction is the reaction between calcium carbonate and hydrochloric acid: CaCO3(s) + 2HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) We could measure the rate of this reaction by measuring the .... ...
Section 4.6: Double Displacement Reactions
... 7. Silver ions are the only metal ions that can be precipitated from a solution containing the C2H3O2− ions. Therefore, a solution such as NaC2H3O2(aq) can be used to precipitate silver ions from a mixture of dissolved metal ions. 8. Answers may vary. Sample answer: Most of the limescale that forms ...
... 7. Silver ions are the only metal ions that can be precipitated from a solution containing the C2H3O2− ions. Therefore, a solution such as NaC2H3O2(aq) can be used to precipitate silver ions from a mixture of dissolved metal ions. 8. Answers may vary. Sample answer: Most of the limescale that forms ...
Kinetics lecture
... Reaction Mechanisms • Reactions may occur all at once or through several discrete steps. • Each of these processes is known as an elementary reaction or elementary process. ...
... Reaction Mechanisms • Reactions may occur all at once or through several discrete steps. • Each of these processes is known as an elementary reaction or elementary process. ...
153KB PDF - Clydeview Academy
... The questions may be answered in any order but all answers are to be written in the spaces provided in this answer book, and must be written clearly and legibly in ink. ...
... The questions may be answered in any order but all answers are to be written in the spaces provided in this answer book, and must be written clearly and legibly in ink. ...
AP `99 Multiple Choice
... 26. When the equation above is balanced and all (B) The oxidation number of H changes from -1 coefficients are reduced to their lowest wholeto +1. number terms, the coefficient for O2(g) is (C) The (A) 6 (B) ...
... 26. When the equation above is balanced and all (B) The oxidation number of H changes from -1 coefficients are reduced to their lowest wholeto +1. number terms, the coefficient for O2(g) is (C) The (A) 6 (B) ...
1999 Advanced Placement Chemistry Exam
... 26. When the equation above is balanced and all (B) The oxidation number of H changes from -1 coefficients are reduced to their lowest wholeto +1. number terms, the coefficient for O2(g) is (C) The (A) 6 (B) ...
... 26. When the equation above is balanced and all (B) The oxidation number of H changes from -1 coefficients are reduced to their lowest wholeto +1. number terms, the coefficient for O2(g) is (C) The (A) 6 (B) ...
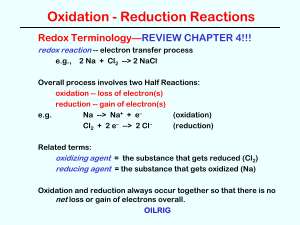
Oxidation and Reduction Reactions
... Deduce redox equations using half-equations. Use H+ and H2O when balancing half-equations in acidic solution. Define the terms oxidizing agent and reducing agent. Identify the oxidizing and reducing agents in redox equations. ...
... Deduce redox equations using half-equations. Use H+ and H2O when balancing half-equations in acidic solution. Define the terms oxidizing agent and reducing agent. Identify the oxidizing and reducing agents in redox equations. ...
GQ2613291336
... distilled water at 298K, and the conductivity cell was fitted with a rubber stopper and the conductivity readings from conductivity meter (C) were recorded (the conductivity of distilled water as a blank was excluded). The other neck of the flask (A) connected by a rubber tube to the flask (I) which ...
... distilled water at 298K, and the conductivity cell was fitted with a rubber stopper and the conductivity readings from conductivity meter (C) were recorded (the conductivity of distilled water as a blank was excluded). The other neck of the flask (A) connected by a rubber tube to the flask (I) which ...
Chapter 1: Fundamental Concepts
... • The transfer of electrons between species, meaning that one species is oxidized and one is reduced. The two processes will always occur together. • When has a redox reaction occurred? – If there is a change in the oxidation state of any element in the reaction, a redox reaction has happened. – Rem ...
... • The transfer of electrons between species, meaning that one species is oxidized and one is reduced. The two processes will always occur together. • When has a redox reaction occurred? – If there is a change in the oxidation state of any element in the reaction, a redox reaction has happened. – Rem ...
Step by Step Stoichiometry
... needed to react with 1.23 grams of sulfur? (You should have the balanced reaction and mole ratios from the previous practice problems) Limiting Reactant (sometimes called limiting reagent): ...
... needed to react with 1.23 grams of sulfur? (You should have the balanced reaction and mole ratios from the previous practice problems) Limiting Reactant (sometimes called limiting reagent): ...
Document
... • The above law, however, does not imply that the absorbed light must always result into chemical reaction. The absorbed light may simply bring about phenomena such as fluorescence, phosphorescence etc., Similarly, the absorbed light energy may be simply converted into thermal energy e.g. in case o ...
... • The above law, however, does not imply that the absorbed light must always result into chemical reaction. The absorbed light may simply bring about phenomena such as fluorescence, phosphorescence etc., Similarly, the absorbed light energy may be simply converted into thermal energy e.g. in case o ...
Chemistry in Society - Cathkin High School
... Looking at the quantities of reactants from step 1 there is not enough oxygen to allow all of the methane to react therefore some methane will be left over at the end. The methane is said to be in excess and the oxygen will therefore determine the quantity of carbon dioxide produced. ...
... Looking at the quantities of reactants from step 1 there is not enough oxygen to allow all of the methane to react therefore some methane will be left over at the end. The methane is said to be in excess and the oxygen will therefore determine the quantity of carbon dioxide produced. ...
CHEM 30 REDOX
... agent. Watch for acids ( H +) Also water H2O Write the reduction half reaction, as written in the data book. Write the oxidation half reaction, reverse the equation in the data book. Balance number of electrons. Add the two half reactions together to form the ...
... agent. Watch for acids ( H +) Also water H2O Write the reduction half reaction, as written in the data book. Write the oxidation half reaction, reverse the equation in the data book. Balance number of electrons. Add the two half reactions together to form the ...
Chemical Kinetics Mac 2011
... • All elementary processes are reversible and may reach a steady-state condition. In the steady state the rates of the forward & reverse processes become equal. • One elementary process may occur much more slower than all the others. In this case, it determines the rate at which the overall reaction ...
... • All elementary processes are reversible and may reach a steady-state condition. In the steady state the rates of the forward & reverse processes become equal. • One elementary process may occur much more slower than all the others. In this case, it determines the rate at which the overall reaction ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.