Notes Set 1
... The table you have in front of you is a much more complete list than the one you made in the lab. It will be provided for you on the final exam. It is important that you know how to use this table. As you can see, there are several reactions that simply involve the transfer of electrons from an ion ...
... The table you have in front of you is a much more complete list than the one you made in the lab. It will be provided for you on the final exam. It is important that you know how to use this table. As you can see, there are several reactions that simply involve the transfer of electrons from an ion ...
ppt - ChemConnections
... Examine the image above and select the correct statement below. A)NaHCO3 is the limiting reagent in the yellow and blue. B)HCl is definitely not the limiting reagent in all three cases. C)Equal numbers of moles of HCl and NaHCO3 may have reacted ...
... Examine the image above and select the correct statement below. A)NaHCO3 is the limiting reagent in the yellow and blue. B)HCl is definitely not the limiting reagent in all three cases. C)Equal numbers of moles of HCl and NaHCO3 may have reacted ...
Lecture Resource ()
... • Some amines are heterocyclic compounds (or heterocycles) • Most drugs, vitamins, and many other natural products are heterocycles • A natural product is a compound synthesized by a plant or an animal ...
... • Some amines are heterocyclic compounds (or heterocycles) • Most drugs, vitamins, and many other natural products are heterocycles • A natural product is a compound synthesized by a plant or an animal ...
Effect Of Convection For Gaseous Hydrochloride
... using oxygen atmosphere in presence of lime, C is removed as CO gas, Si, P and S form slag. Small percentage of Fe is oxidized as well, and leaves the batch together with oxidized heavy metals. It is collected as a dust. For the steel production, 6 millions ton/year in the Czech, 100 000 t of the du ...
... using oxygen atmosphere in presence of lime, C is removed as CO gas, Si, P and S form slag. Small percentage of Fe is oxidized as well, and leaves the batch together with oxidized heavy metals. It is collected as a dust. For the steel production, 6 millions ton/year in the Czech, 100 000 t of the du ...
Final Exam SG Part 1 (Unit 5).
... b. How many grams of bromine are needed to completely react with 4.60 X 1023 molecules of sodium hypoiodite? ...
... b. How many grams of bromine are needed to completely react with 4.60 X 1023 molecules of sodium hypoiodite? ...
Stoichiometry Notes
... We use ratios to set up equations. For example, if we knew the number of moles of CH 4 that undergoes a chemical reaction and we needed to find out information regarding the water, we would use the following ratio: 1 mole CH4 -------------------------2 moles of H2O ...
... We use ratios to set up equations. For example, if we knew the number of moles of CH 4 that undergoes a chemical reaction and we needed to find out information regarding the water, we would use the following ratio: 1 mole CH4 -------------------------2 moles of H2O ...
Practice Test Stoichiometry
... 13.) Which of the following compounds has the same percent composition by mass as styrene, C8H8? A) acetylene, C2H2 B) benzene, C6H6 C) cyclobutadiene, C4H4 D) -ethyl naphthalene, C12H12 E) all of these 14.) You take an aspirin tablet (a compound consisting solely of carbon, hydrogen, and oxygen) w ...
... 13.) Which of the following compounds has the same percent composition by mass as styrene, C8H8? A) acetylene, C2H2 B) benzene, C6H6 C) cyclobutadiene, C4H4 D) -ethyl naphthalene, C12H12 E) all of these 14.) You take an aspirin tablet (a compound consisting solely of carbon, hydrogen, and oxygen) w ...
Carefully detach the last page. It is the Data Sheet.
... convert carbon dioxide, water and energy into glucose and oxygen. The process of photosynthesis can be represented by the following chemical equation. ...
... convert carbon dioxide, water and energy into glucose and oxygen. The process of photosynthesis can be represented by the following chemical equation. ...
Paired with Lecture
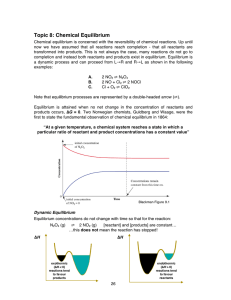
... • We just studied Phase Diagrams which are thermodynamic maps which tell us the equilibrium phases present at any specific combination of temperature, pressure, and composition • These phase diagrams are based on the concept of Gibbs Free Energy, DG, which we have briefly introduced before: DG is ...
... • We just studied Phase Diagrams which are thermodynamic maps which tell us the equilibrium phases present at any specific combination of temperature, pressure, and composition • These phase diagrams are based on the concept of Gibbs Free Energy, DG, which we have briefly introduced before: DG is ...
Document
... Ch 9 Test: Chemical Quantities Round final answers to the correct number of significant figures. Balance all equations as necessary. Show work where indicated. 1. Given the balanced equation 2A + 3B 5C + 4D If 3.50 moles of A react, how many moles of product C can be formed? 2. Given the balanced ...
... Ch 9 Test: Chemical Quantities Round final answers to the correct number of significant figures. Balance all equations as necessary. Show work where indicated. 1. Given the balanced equation 2A + 3B 5C + 4D If 3.50 moles of A react, how many moles of product C can be formed? 2. Given the balanced ...
lec09 - McMaster Chemistry
... Non-exothermic spontaneous reactions But many spontaneous reactions or processes are endothermic . . . NH4NO3(s) + heat NH4+ (aq) + NO3- (aq) Hsol = +25.7 kJ/mol or have H = 0 . . . ...
... Non-exothermic spontaneous reactions But many spontaneous reactions or processes are endothermic . . . NH4NO3(s) + heat NH4+ (aq) + NO3- (aq) Hsol = +25.7 kJ/mol or have H = 0 . . . ...
balancing chemical equations worksheet
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
CHEM230P1_06_2014_Y_P1
... The pressure in the container is now increased by decreasing the volume of the container. Explain how the composition of A and B will change during this process and also state whether the equilibrium constant, KP, will increase, decrease or stay the same. ...
... The pressure in the container is now increased by decreasing the volume of the container. Explain how the composition of A and B will change during this process and also state whether the equilibrium constant, KP, will increase, decrease or stay the same. ...
No Slide Title
... concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. ________________ – the point at which the reaction is complete _______________ – substance that changes color at (or near) the equivalence point ...
... concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. ________________ – the point at which the reaction is complete _______________ – substance that changes color at (or near) the equivalence point ...
Teacher Background - Online Learning Exchange
... balanced chemical equations as a basis for calculating how much reactant is needed or how much product will be formed in a reaction. When you know the quantity of one substance in a reaction, you can calculate the quantity of any other substance consumed or produced in the reaction. Quantity usually ...
... balanced chemical equations as a basis for calculating how much reactant is needed or how much product will be formed in a reaction. When you know the quantity of one substance in a reaction, you can calculate the quantity of any other substance consumed or produced in the reaction. Quantity usually ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.