PDF File
... lone-pair electrons that can accept hydrogen bonds but cannot donate hydrogen bonds, the higher reactivity of the substrate with 2′-OH than 2′-F at U(–1) suggests that hydrogen-bond donation from the 2′-OH of U(–1) might be important. A hydrogen bond from this 2′-OH to the neighboring 3′-oxygen is t ...
... lone-pair electrons that can accept hydrogen bonds but cannot donate hydrogen bonds, the higher reactivity of the substrate with 2′-OH than 2′-F at U(–1) suggests that hydrogen-bond donation from the 2′-OH of U(–1) might be important. A hydrogen bond from this 2′-OH to the neighboring 3′-oxygen is t ...
1.6 Energy changes in chemical reactions
... In the field of renewable energy, dye-sensitized solar cells are being developed that will provide a cheaper alternative to silicon-based products. Light-harvesting dyes containing ruthenium are adsorbed onto a thin film of titanium dioxide (TiO2) nanoparticles in contact with a redox electrolyte. T ...
... In the field of renewable energy, dye-sensitized solar cells are being developed that will provide a cheaper alternative to silicon-based products. Light-harvesting dyes containing ruthenium are adsorbed onto a thin film of titanium dioxide (TiO2) nanoparticles in contact with a redox electrolyte. T ...
Reaction Kinetics - National Open University of Nigeria
... CHM 407: Reaction Kinetics concerns with the speed or rates of chemical reactions. The study of reaction rates allows for the prediction of how fast it will take a reaction mixture to reach equilibrium. It also account for how the reaction rate would be optimised by controlling certain factors such ...
... CHM 407: Reaction Kinetics concerns with the speed or rates of chemical reactions. The study of reaction rates allows for the prediction of how fast it will take a reaction mixture to reach equilibrium. It also account for how the reaction rate would be optimised by controlling certain factors such ...
Stoichiometry Notes
... If the number of equivalence of both the reactants are different then reactant with the lesser number of equivalence will be the limiting reagent. Application of equivalent concept : It is used in acid base titration, back titration and double titration, similarly in redox titration. Equivalent conc ...
... If the number of equivalence of both the reactants are different then reactant with the lesser number of equivalence will be the limiting reagent. Application of equivalent concept : It is used in acid base titration, back titration and double titration, similarly in redox titration. Equivalent conc ...
Η - Knockhardy
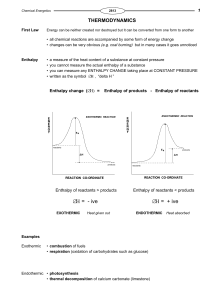
... Calculate the standard enthalpy change for the following reaction, given that the standard enthalpies of formation of water, nitrogen dioxide and nitric acid are -286, +33 and -173 kJ mol-1 respectively. [ the value for oxygen is ZERO as it is an element ] 2H2O(l) applying Hess’s Law ... ...
... Calculate the standard enthalpy change for the following reaction, given that the standard enthalpies of formation of water, nitrogen dioxide and nitric acid are -286, +33 and -173 kJ mol-1 respectively. [ the value for oxygen is ZERO as it is an element ] 2H2O(l) applying Hess’s Law ... ...
Slide 1
... I. Oxidation & Reduction -a substance which ________ oxidizes another substance by ________ accepting its ________ electrons is called an ________ oxidizing _____, agent which is also reduced the substance that is _______ -a substance which _______ reduces another substance by ______ losing ________ ...
... I. Oxidation & Reduction -a substance which ________ oxidizes another substance by ________ accepting its ________ electrons is called an ________ oxidizing _____, agent which is also reduced the substance that is _______ -a substance which _______ reduces another substance by ______ losing ________ ...
word - My eCoach
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
Last Name Professor BEAMER First Name
... Solution/Explanation: You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
... Solution/Explanation: You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
Chp 5 Circle the correct answer Consider three 1
... a) Yes, ΔE = 0 at all times, which is why q = -w. b) No, ΔE does not always equal zero but this is only due to factors like friction and heat. c) No, ΔE does not always equal zero because it refers to the system’s internal energy which is affected by heat and work. d) No, ΔE never equals zero becaus ...
... a) Yes, ΔE = 0 at all times, which is why q = -w. b) No, ΔE does not always equal zero but this is only due to factors like friction and heat. c) No, ΔE does not always equal zero because it refers to the system’s internal energy which is affected by heat and work. d) No, ΔE never equals zero becaus ...
File
... 1 Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2 Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3 Balanced Chemical e ...
... 1 Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2 Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3 Balanced Chemical e ...
- Kendriya Vidyalaya No. 2 Raipur
... 1 Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2 Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3 Balanced Chemical e ...
... 1 Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2 Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3 Balanced Chemical e ...
Oxidation-Reduction Reactions - An Introduction to Chemistry
... no ions are formed. Nitrogen monoxide is a molecular compound, and the bonds between the nitrogen and the oxygen are covalent bonds, in which electrons are shared. Because the oxygen atoms attract electrons more strongly than nitrogen atoms, there is a partial transfer of electrons from the nitrogen ...
... no ions are formed. Nitrogen monoxide is a molecular compound, and the bonds between the nitrogen and the oxygen are covalent bonds, in which electrons are shared. Because the oxygen atoms attract electrons more strongly than nitrogen atoms, there is a partial transfer of electrons from the nitrogen ...
PRACTICE EXAM 1-C
... You are given a mixture of barium hydroxide, Ba(OH)2, and strontium hydroxide, Sr(OH)2. You dissolve this mixture in water and titrate it with hydrochloric acid. Complete neutralization of the mixture requires 96.0 mL of 1.50 M HCl. (Note that Sr(OH)2 is a strong base that reacts with HCl in the sam ...
... You are given a mixture of barium hydroxide, Ba(OH)2, and strontium hydroxide, Sr(OH)2. You dissolve this mixture in water and titrate it with hydrochloric acid. Complete neutralization of the mixture requires 96.0 mL of 1.50 M HCl. (Note that Sr(OH)2 is a strong base that reacts with HCl in the sam ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.